Impact of Alemtuzumab Induction on Pancreas Transplant Outcomes
Abdolreza Haririan1, Ian Booth2, Brian Masters2, Jillian Casale2, Joseph Scalea3, Cinthia Drachenberg4.
1Medicine, University of Maryland, Baltimore, MD, United States; 2Pharmacy, University of Maryland Medical Center, Baltimore, MD, United States; 3Surgery, University of Maryland, Baltimore, MD, United States; 4Pathology, University of Maryland, Baltimore, MD, United States
The impact of the current choices of induction therapy for pancreas transplantation (PT), particular alemtuzumab, on transplant outcomes have not been well studied.
We conducted a single center, retrospective cohort study comparing pancreas transplant outcomes including patinet survival, pancreas allograft survival, and biopsy-proven acute rejection in both solitary and combined PT recipients receiving basiliximab, rATG, or alemtuzumab induction.
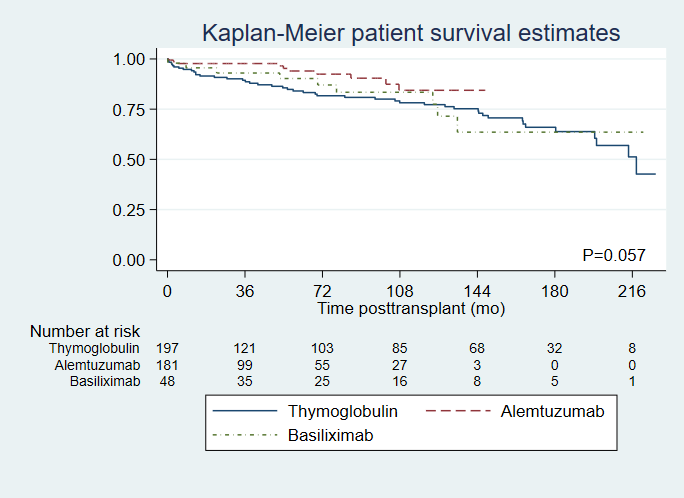
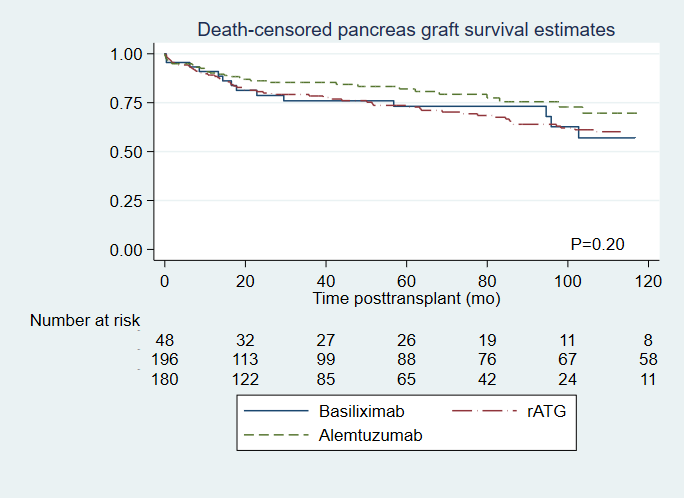
A total of 428 PTs transplanted between January 2000 and May 2018 were included in the final analysis (48 in basiliximab group, 199 in rATG group and 181 in alemtuzumab group). The overall patient survival rate was significantly higher in the alemtuzumab group compared to the rATG group (adjusted HR 0.45, 95%CI 0.22-0.94, P=0.033), while basiliximab was comparable to rATG (HR 0.89, 95% CI 0.43-1.82; p=0.74) [Figure 1]. Death-censored pancreas graft loss was similar between the groups (HR 0.7, 95% CI 0.79-2.86; p=0.11 for alemtuzumab, and HR 1.08, 95% CI 0.62-1.87; p=0.8 for basiliximab) [Figure 2]. Incidence of BPAR was similar between the groups (HR 0.82, 95% CI 0.38-1.77; p=0.61 for alemtuzumab, and HR 0.61, 95% CI 0.36-1.04; p=0.07 for basiliximab). However, more severe rejection was observed more frequently in rATG group (41.7% versus 23.8% in alemtuzumab, and none in basiliximab groups). The observed infectious complications were comparable between the induction groups.
Our study suggests that alemtuzumab is a safe and effective induction agent that can be utilized for PT recipients to achieve outcomes comparable to other available agents, with a possible survival advantage for the patients.