
High glucose concentration increases KATP channel activity but suppresses mitochondrial respiration ability in insulin-producing cells regenerated from stem cells
Chencheng Wang1,3, Shadab Abadpour1,2,3, Alexandra Aizenshtadt1, Simona Chera4, Luiza Ghila4, Helge Ræder4,5, Hanne Scholz1,2,3.
1Hybrid Technology Hub, Center of Excellence, University of Oslo, Oslo, Norway; 2Institute for Surgical Research, Oslo University Hospital, Oslo, Norway; 3Department of Transplant Medicine, Oslo University Hospital, Oslo, Norway; 4Department of Clinical Science, University of Bergen, Bergen, Norway; 5Department of Pediatrics, Haukeland University Hospital, Bergen, Norway
Introduction: Beta cells have been successfully differentiated from human pluripotent stem cells (hPSCs) by mimicking the pancreatic developmental process, provide a virtually unlimited source of cells for curative diabetes treatment. Current protocols to generate hPSC-derived insulin-producing cells (IPCs) consist of exogenous non-physiological high glucose levels (≥20 mM) for the final steps. These glucose levels have been considering a key contributor to cell fate definition and maturation. However, long-term exposure to high glucose levels has been linked to beta-cell dedifferentiation and less robust glucose-stimulated insulin secretion (GSIS) in human islets in vitro. This study aims to investigate hiPSCs to IPCs differentiation under physiological glucose (5.5mM) compared with a high glucose level (20mM) after the pancreas progenitor stage.
Methods: The hiPSC lines (Babk2, WTC11, INSULIN-H2B-Cherry (InsCherry)) were differentiated following the seven-stages protocol (Rezania et al., 2014) illustrated in Figure 1A. Stage 6 cells were differentiated under 20mM or 5.5mM glucose, followed by culturing in stage 7 media contains 5.5mM glucose, and then cultured in the same media in suspension for 7-10 days (Stage 7+ cells) until collecting for GSIS assay, oxygen consumption rate (OCR) measurement, and calcium flux analyzing. Stage 6 cells were analyzed by immunofluorescences staining. Stage 6 and Stage 7+ cells were collected for gene expressions and mitochondria contents analysis.
Results: Stage 6 Cells (Babk2 and WTC11) differentiated under 20mM glucose showed significantly reduced NKX6.1 gene expression and co-localization of PDX1+/NKX6.1+ (Figures 1B and C, P<0.001, n=5). For osmotic pressure control, the cells under 5.5mM media were supplemented with mannitol which supports a direct effect of the glucose (Figure 1C). The GSIS showed no functionality in either 20 mM or 5.5mM glucose differentiated cells (Figure 1E). But differentiated InsCherry-iPS in 20mM glucose obtained functionality demonstrated by the GSIS (Figures 1D and F). The disallowed gene LDHA was more successfully suppressed in 20mM condition (Figure 1G), and KATP channel subunits gene ABCC8 was increased in high glucose-induced differentiation from stage 6 to 7+ (Figure 1H). Calcium flux analysis showed an improved KATP channel activity in high glucose-induced cells (Figure 1I). The mitochondrial biogenesis was suppressed temporally in stage 6 cells exposed to 20 mM (Figure 1J). The OCR analysis suggests that high glucose negatively impacts mitochondrial spare respiratory capacity (Figure 1K).
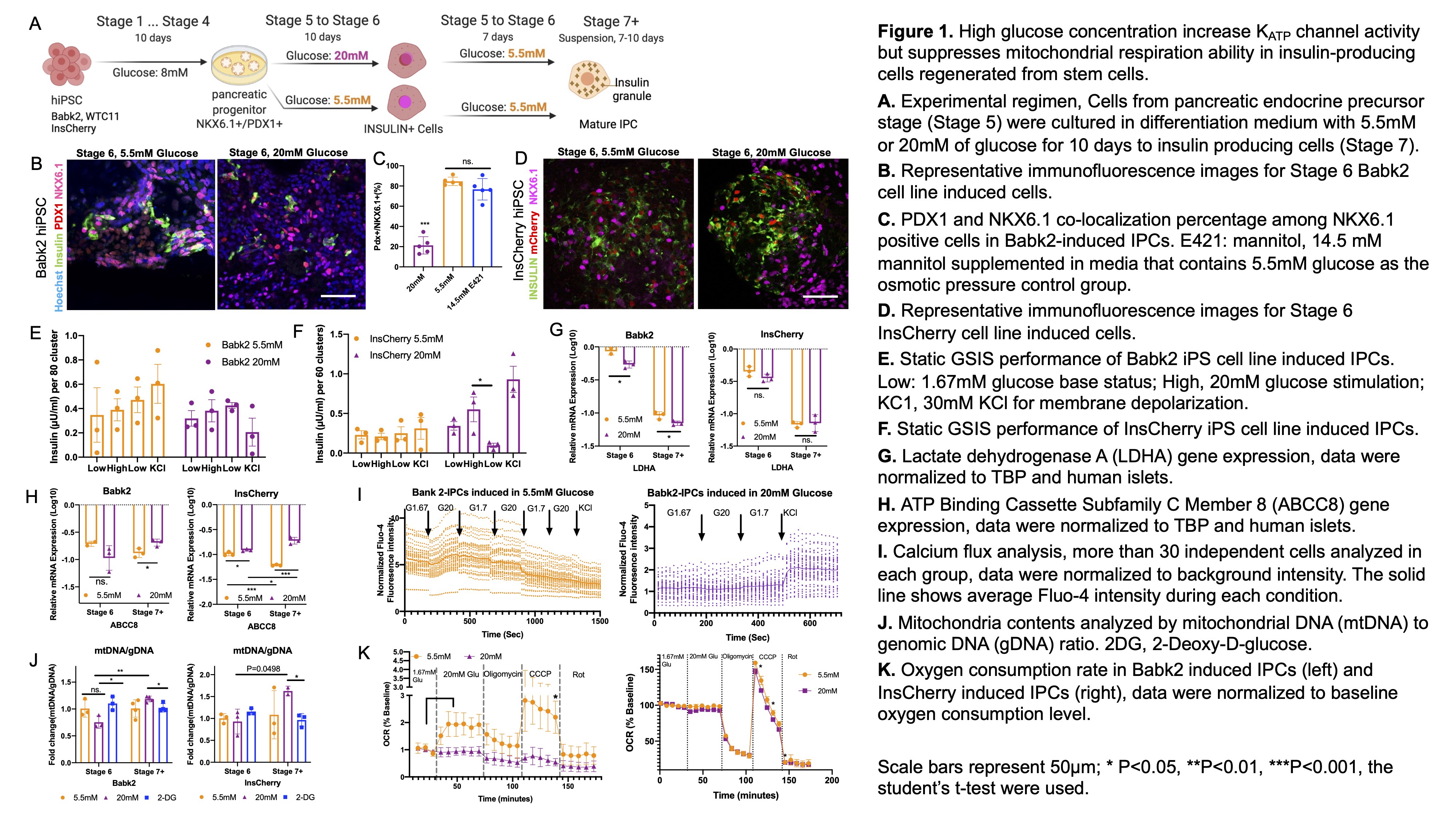
Conclusion: The impact of high glucose levels on IPC differentiation is complex and cell line dependent. We found that high glucose level benefits KATP channel activity and helps obtain functionality of the differentiated cells. In contrast, it suppresses mitochondrial respiration ability. Our study supports the use of high glucose for IPC generation, but this seems to be on behalf of mitochondrial respiration ability. New and better solutions to bypass and/or rescue the side-effect from high glucose induction will be needed.