A human pancreatic hydrogel optimized for 3-D modeling of the islet microenvironment
Daniel Tremmel1, Austin K Feeney1, Samantha A Mitchell1, Sakar Gupta1, Sara D Sackett1, Jon S Odorico1.
1Surgery - Transplantation, University of Wisconsin, Madison, WI, United States
Introduction: Islet isolation results in damage to the extracellular matrix (ECM) which limits islet survival and function in vitro. We developed a human pancreas ECM hydrogel (hP-HG) that is compatible with islet culture and transplantation, which reconstructs the islet ECM environment to extend islet viability and function. This platform enables the study of the important interactions between islets and their microenvironment.
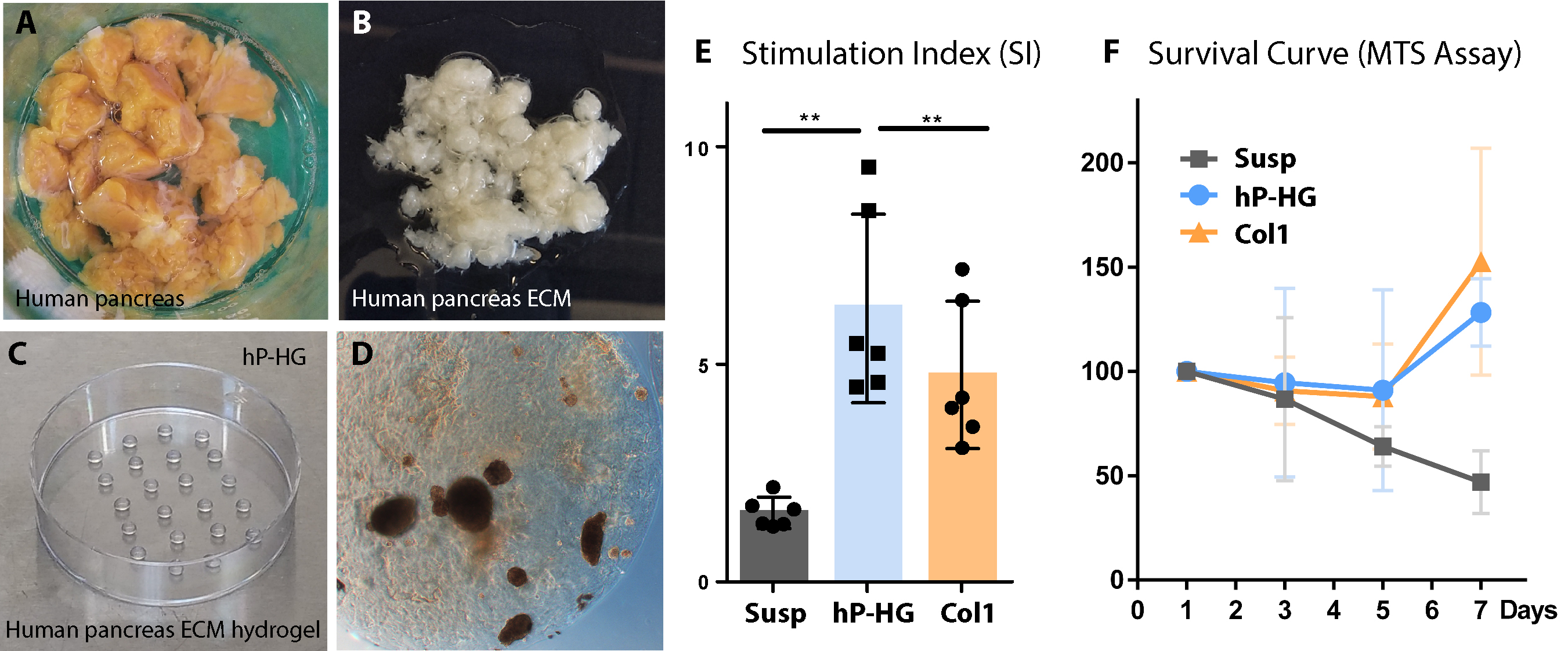
Methods: hP-HG was prepared by pancreas decellularization (Fig 1A-B), solubilization of the ECM, and neutralization to form a gel (Fig 1C). Human islets were cultured in suspension (susp), embedded in hP-HG droplets (Fig 1D), or purified Collagen 1 (Col1). Islet survival was assessed with an MTS assay, function was assessed through static and perifusion GSIS, mitochondrial function assessed through respirometry, and islet architecture was assessed with IF staining and 3D tissue analysis.
Results: Islets cultured in hP-HG had a significantly improved stimulation index (SI= 6.3) compared to islets from the same donors cultured in susp (SI= 1.6) (Fig 1E). The increased SI was reflective of a significantly lower basal insulin secretion level in hP-HG culture compared to susp. From 7-14 days of culture, the basal insulin secretion rate (at 2.8 mM glucose) in susp culture was found to significantly increase, indicative of islet dysfunction, while the basal secretion in hP-HG culture remained stable and low. At 7 days of culture, islets in hP-HG had near 100% survival, while islets in susp had only 50% survival (Fig 1F). Islet mitochondrial function was found to have a higher maximal respiratory capacity in hP-HG, compared to susp.
Endocrine and non-endocrine cell arrangements were found to be significantly different among islets in susp compared to hP-HG culture. In susp, endocrine cells moved outward, with the majority of β cells being in the mantle, while endothelial cells and fibroblasts moved toward the center of the islets. Islets in hP-HG, on the other hand, more closely resembled in situ islets, with the β cells and endothelial cells more evenly distributed across the islet, and fibroblasts almost exclusively at the mantle (Fig 2).
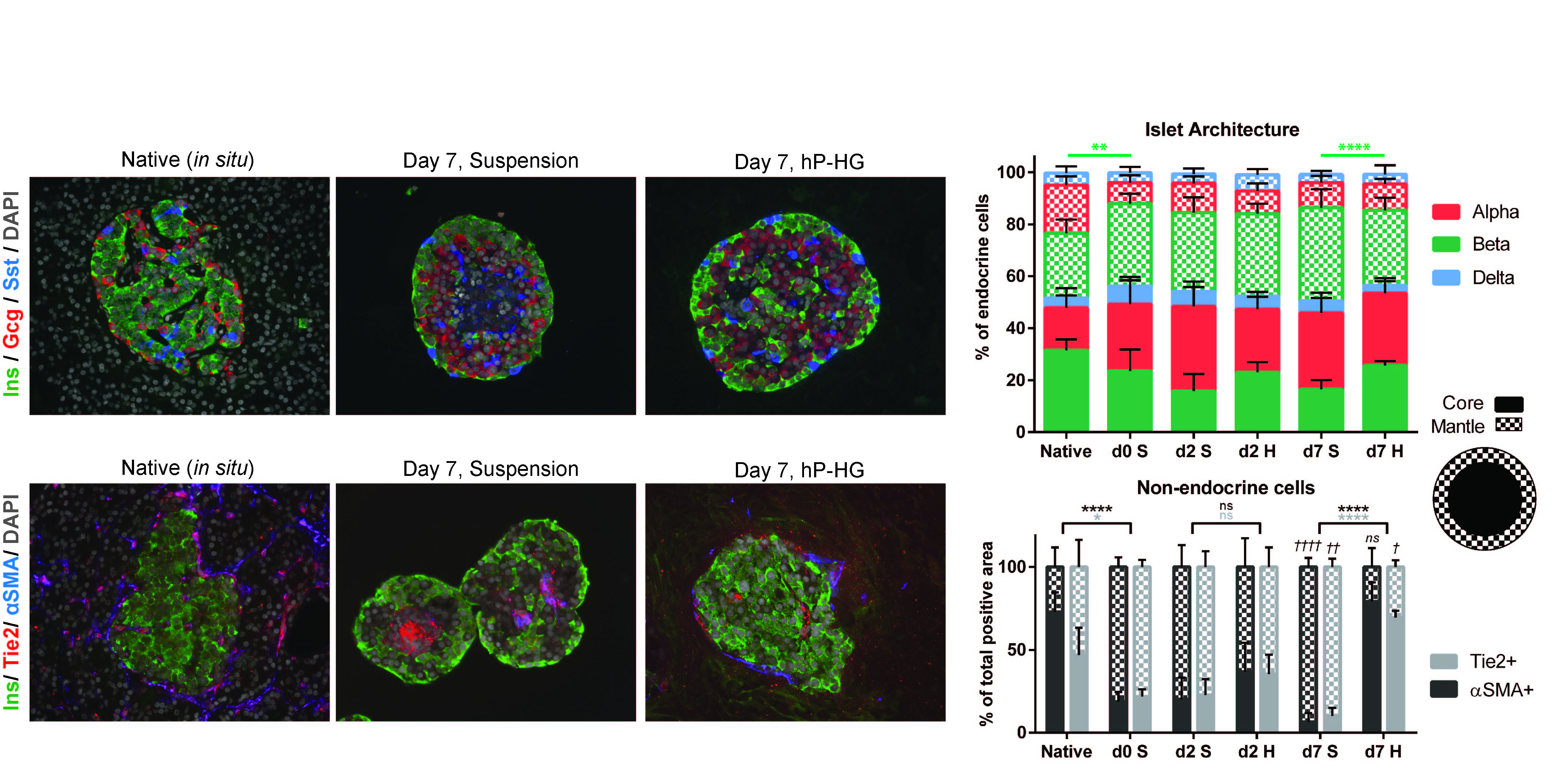
Conclusions: We demonstrate that pancreatic ECM co-culture improves the survival and glucose responsiveness of human islets in vitro. Replicating the native human pancreas ECM environment with hP-HG had significantly higher SI and improved overall insulin secretion compared to Col1 culture; this suggests a role for pancreas-specific ECM in islet health. Correlating with improved function, islets in hP-HG maintain native cell arrangements, while islets cultured in suspension over time become dysmorphic, with abnormal central nodules of endothelial cells and fibroblasts, and a β cell-enriched mantle. The hP-HG platform is useful for future studies incorporating other elements of the islet microenvironment, and could potentially enhance islet survival following transplantation.