Neonatal porcine Pseudo-Islets - a standardized and scalable source for islet encapsulation?
Undine Schubert1,2, Christian Cohrs2, Elisabeth Kemter3, Barbara Ludwig1,2.
1Department of Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany; 2Paul Langerhans Institute Dresden, Helmholtz Center Munich at The University Hospital Dresden, Dresden, Germany; 3Department of Molecular Animal Breeding and Biotechnology, Ludwig Maximilian University, Dresden, Germany
Introduction: In recent years, numerous improvements in islet isolation strategies and the introduction of a tailor-made immunosuppressive and anti-inflammatory therapy regimen have positively influenced the clinical outcome of human pancreatic islet transplantation. In spite of these successes, however, considerable restrictions remain, which make a broader application of this therapeutic approach difficult. In order to circumvent the restriction caused by a lack of donor organs, the development of a macroencapsulation device with xenogeneic islets would be ideal, which offers sufficient immune isolation while maintaining the regulated islet function. Key factors for optimal islet material in the encapsulation setting are to provide intact islet clusters with high purity and homogeneity and a minimal size distribution in order to reduce oxygen demand, maximize applicable density and optimize diffusion characteristics. Therefore, the focus of this work is the optimization and standardization of the islet material by using so-called pseudo-islets.
Method: With the help of a new type of spherical culture technology (Sphericalplate® 5D from KUGELMEIERS®) we were able to generate pseudo-islets with a defined size.
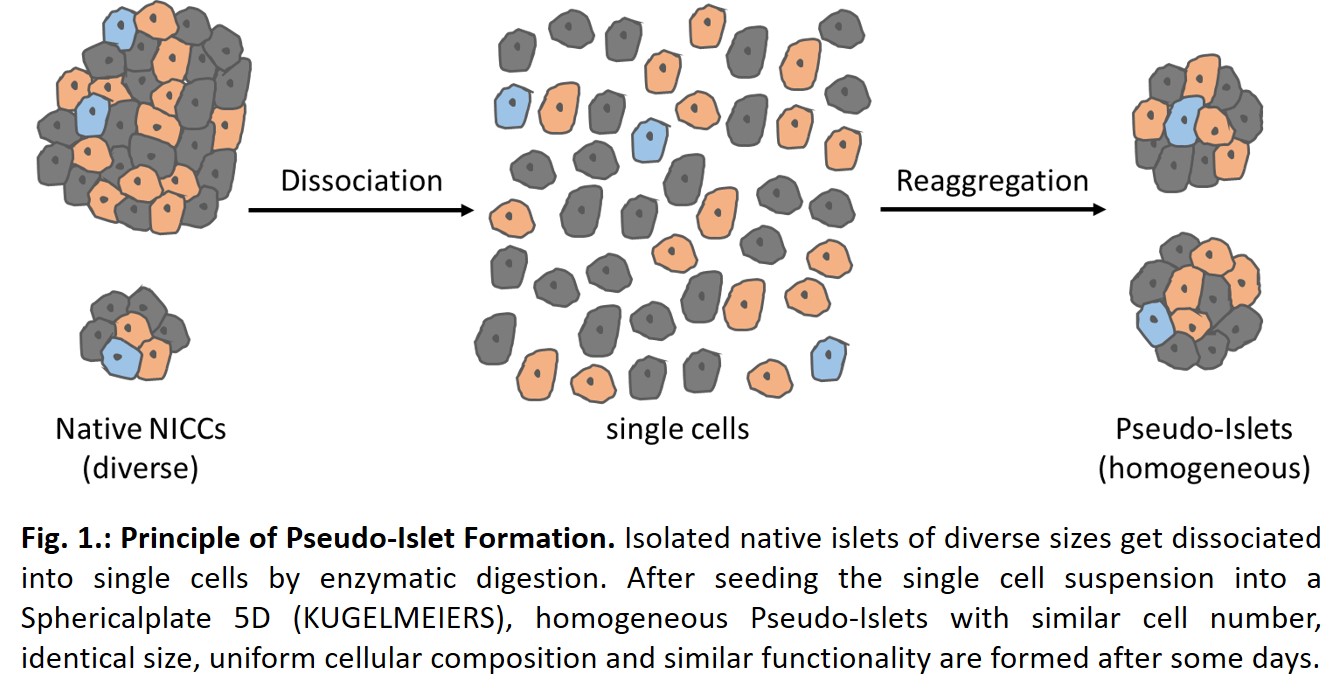
For this purpose, islets were dissociated into single cells and seeded in densities of 300, 600, 900, 1200 or 1500 cells / microwell to identify the optimal cluster size. To this end, we used our established quality control assessment to compare pseudo-islets to native control NICCs. Based on long-term considerations of safety, efficacy, and cost-effectiveness, we investigated porcine neonatal islet like cell clusters (NICCs) as an alternative cell source to adult pig islets.
Results: In our study, we were able to generate homogeneous, standardized pseudo-islets from porcine NICCs with a scalable size between 73-132 µm.
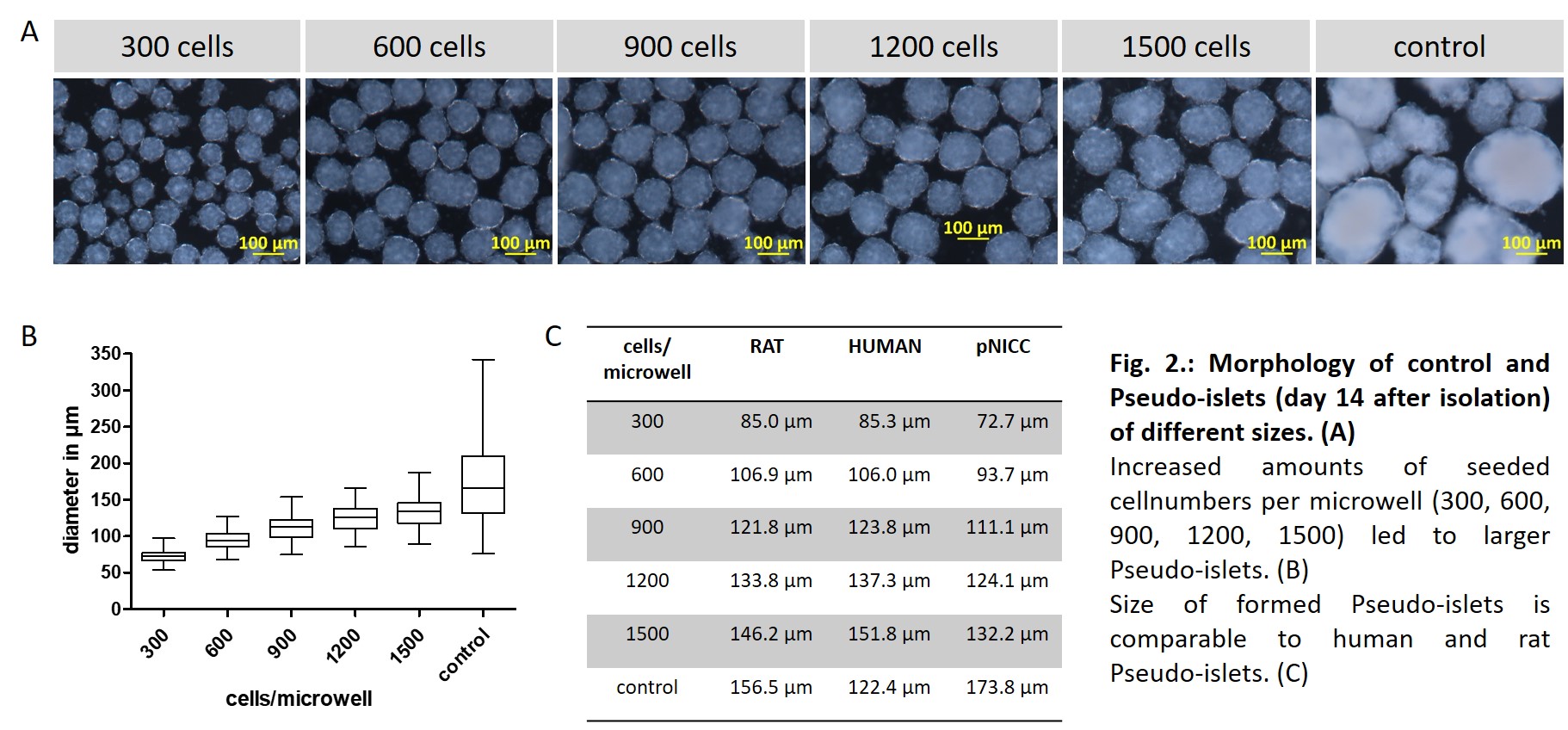
The size of the pseudo-islets formed could be defined by the number of individual cells seeded per microwell. Pseudo-NICCs showed improved vitality compared to native control islets. In addition, we observed that control and pseudo-islets react dynamically to glucose and begin to secrete insulin after a glucose challenge. The cell composition of the islets was determined by triple immunostaining against insulin, glucagon and somatostatin and showed a comparable proportion of endocrine cells in porcine pseudo-NICCs compared to native NICCs. After transplantation into the anterior chamber of the eye, pseudo-NICCs showed correct growth and less cell loss compared to the control.
Conclusion: We were able to show the potential of this culture technology to produce scalable and homogeneous islet preparations. Particularly in the encapsulation environment, where the rate of oxygen consumption and diffusion properties are essential for function and maximizing density, the creation of pseudo-islets with optimal dimensions may have significant beneficial effects on islet survival after transplantation and could help to overcome nutrient diffusion limitations.