
Outcomes of Simultaneous Kidney-Pancreas Transplantation in Patients with Type-1 and Type-2 Diabetes Mellitus
Muhammad Saad Naseer1, Hosein Shokouh-Amiri1, Gazi Zibari1, Donnie Aultman1, Srijan Tandukar1, Neeraj Singh1.
1Kidney and Pancreas Transplantation, John C. McDonald Regional Transplant Center - Willis Knighton Health System, Shreveport, LA, United States
Background: UNOS approved simultaneous kidney-pancreas transplantation (SKPT) for type-II diabetes mellitus (DM) in Oct 2014. In Jan 2015, we actively listed type-II DM patients with ESRD or e-GFR ≤ 20 ml/min/m2 for SKPT if they met the following criteria: age < 55 years, on insulin ≤ 1 unit/kg body weight, and a maximum allowable body mass index (BMI ≤ 30 kg/m2). The two major advantages for the type-II DM patients receiving SKPT as compared with kidney transplant alone are a much shorter waiting time and usually a better-quality kidney. There is limited data comparing outcomes of SKPT in type-I versus type-II DM. The aim of this study was to measure the change in volume of SPKT since UNOS approved it for type-II DM patients and compare death-censored 5-year graft and patient survival between type-I and type-II DM patients undergoing SKPT.
Methods: We conducted a retrospective chart-review study on SKPT patients from Jan 2010 to Nov 2020 and collected data on patient demographics such as age, gender, and race. BMI, c-peptide, HbA1c, and e-GFR were noted pre-transplant and 1-year post-transplant. Outcome variables included volume of SKPT pre-and post-approval of SKPT for type-II DM patients, death-censored 5-year pancreas and kidney graft survival, and 5-year patient survival. Student’s t-test for continuous variables, Chi-square test for categorical variables, and Mantel-Cox test for survival analysis was done using SPSS.
Results: As compared to type-I DM patients, type-II DM patients were older [47.4 vs 40.2 years, p-value = <0.01] and had higher BMI [32.0 vs 26.7 kg/m2, p-value = <0.01] and eGFR [76.7 vs 62.0 ml/min/1.73m2, p-value = 0.04] 1-year post-transplant (Figure 1). Among 89 SKPT, 18 (all type-I DM) were done before the approval of SKPT in type-II DM patients and 71 (41 type-I and 30 type-II DM) were done after the approval. This translated to an increase in SKPT from 3.6/year to 11.8/year (228% increase). There were no differences in death-censored 5-year kidney and pancreas graft survival and 5-year patient survival (Figure 2).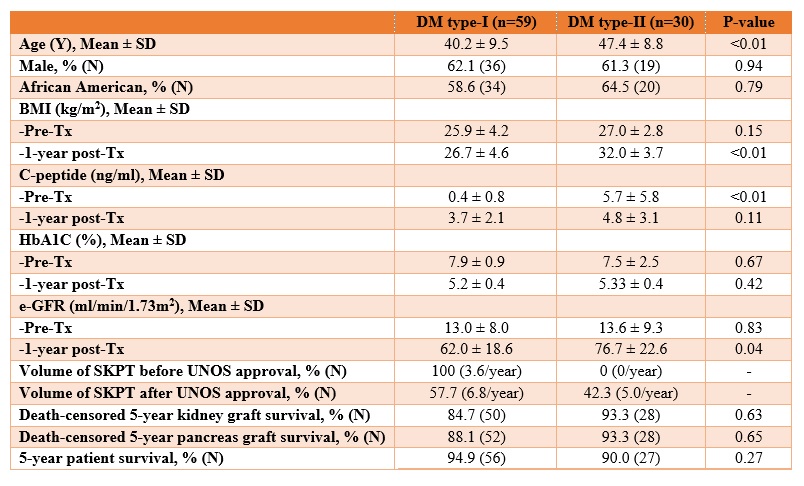
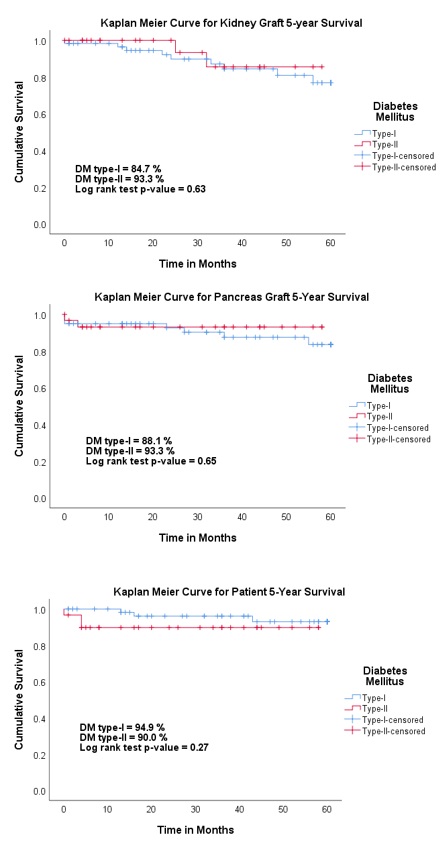
Conclusion: Approval of SKPT for type-II DM by UNOS led to an increase in SKPT with no differences in kidney and pancreas graft survival or patient survival between patients with type-I and type-II DM. Obesity should be carefully screened and managed post-transplantation in type-II DM patients undergoing SKPT.