Long term outcomes after fetal pancreatic stem cell transplantation in diabetes mellitus: data from unified national electronic health system 2014-2019
Abduzhappar Gaipov1, Arnur Gusmanov1, Yesbolat Sakko1, Alpamys Issanov1, Dinara Galiyeva1, Raushan Alibekova1, Kainar Kadyrzhanuly1, Samat Saparbayev2, Zhannat Taubaldiyeva3, Saltanat Tuganbekova3, Antonio Sarria-Santamera1, Zhaxylyk Doskaliyev2, Abay Baigenzhin3.
1Department of Medicine, Nazarbayev University School of Medicine, Nur-Sultan, Kazakhstan; 2Department of Surgery, West Kazakhstan Marat Ospanov Medical University, Aktobe, Kazakhstan; 3Department of Medicine, National Scientific Medical Center, Nur-Sultan, Kazakhstan
Introduction: Fetal pancreatic stem cell transplantations (FPCT) from the aborted fetuses of 16-20 weeks gestation have been approved for the treatment of diabetes mellitus (DM) in a single tertiary research and training hospital in Kazakhstan. Although the immediate short-term beneficial effect was observed, there was a lack of long-term mortality assessment and the risk of diabetes-related complications among patients receiving FPCT compared to patients receiving conventional treatment (CT). We aimed to study long-term all-cause mortality and diabetes-related complications in DM patients who underwent EPCT versus CT using large-scale administrative health data from Unified National Electronic Health System (UNEHS).
Methods: The initial cohort of 102,611 patients who were admitted to the hospital with DM were identified using ICD-10 codes through UNEHS between 2014-2019. Out of them, 504 patients who underwent FPCT were defined using ICD-9 procedure codes. Mortality data and patient-related demographics were linked from the National Population Registry. Identified datasets were cross-checked with the whole UNEHS dataset (n=1,565,093 observations) to ascertain the following complications/comorbidities: coronary heart disease (CHD), stroke, retinopathy, nephropathy, neuropathy, diabetic foot, neoplasms. Based on patients’ baseline characteristics (age, sex, duration of DM, residency, ethnicity, number of hospitalizations, and type of diabetes), the cohort of FPCT patients (n=504) matched with a cohort of CT patients (n=102,611) with a 1:5 ratio by propensity score matching (PSM). The final cohort of 2847 patients (504 with EPCT vs 2,343 with CT) was further evaluated.
Results: After PS matching, baseline characteristics for FPCT and CT groups did not differ. Median (IQR) age was 50.6 (41.5-57.4) years old and the median duration of diabetes accounted for 6.3 (4.7-10.7) years. The cohort consisted of 55.4% females, 75.2% Kazakhs, 85.3% of type 2 DM, and 72.2% of urban residents. The median number of hospitalizations in 2014-2019 was 3 (1-6). The results of diabetes-related events and all-cause mortality presented in table 1. 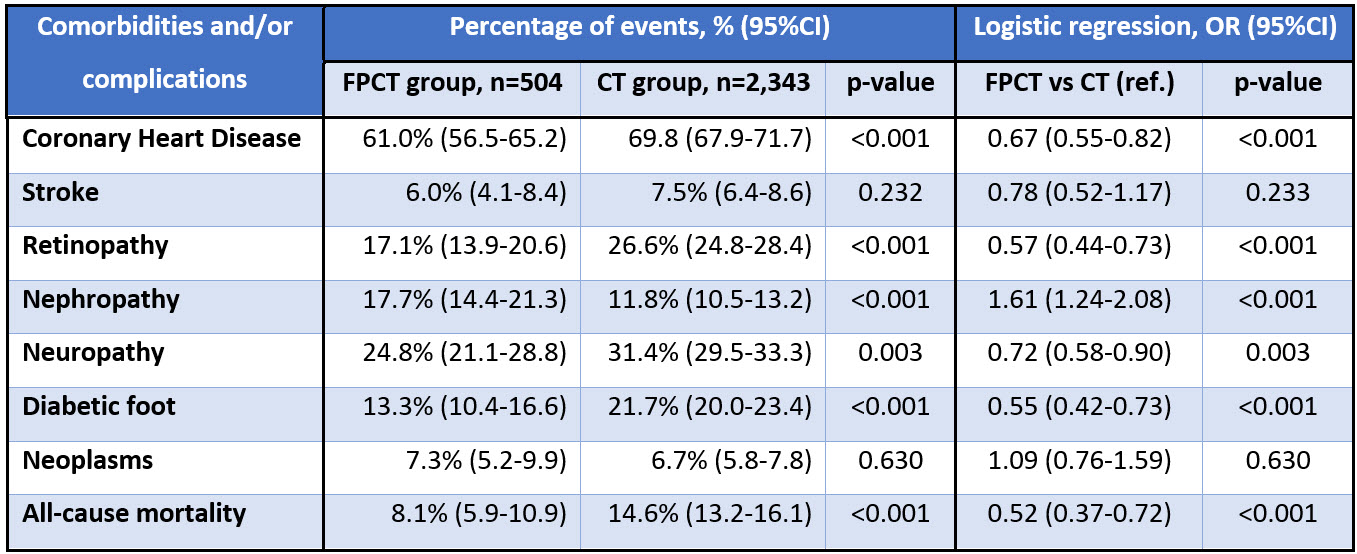 In logistic regression analysis, FPCT group had 48% lower risk of all-cause death compared to CT group (Odds Ratio 0.52; 95%CI: 0.37-0.72; p-value <0.001). Also, those DM patients receiving FPCT compared to the CT group were less likely to experience CHD, diabetic retinopathy, neuropathy, and diabetic foot, however, they were more likely to have diabetic nephropathy. There was no difference between the groups in terms of stroke and cancer incidences. Survival analysis depicted in figure 1.
In logistic regression analysis, FPCT group had 48% lower risk of all-cause death compared to CT group (Odds Ratio 0.52; 95%CI: 0.37-0.72; p-value <0.001). Also, those DM patients receiving FPCT compared to the CT group were less likely to experience CHD, diabetic retinopathy, neuropathy, and diabetic foot, however, they were more likely to have diabetic nephropathy. There was no difference between the groups in terms of stroke and cancer incidences. Survival analysis depicted in figure 1. 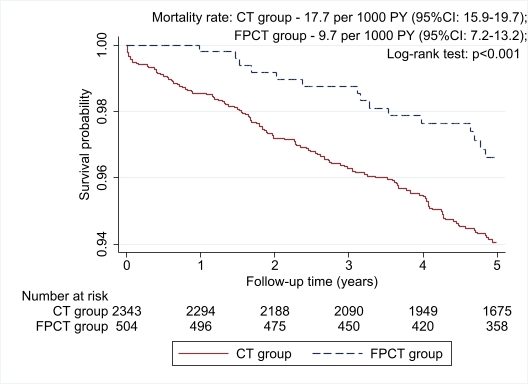
Conclusion: Findings suggest that patients receiving FPCT had a 48% lower risk of all-cause death. We also reveal better outcomes for the FPCT group regarding CHD, retinopathy, nephropathy, neuropathy, and diabetic foot, however, the EPCT group had a higher risk of nephropathy. Further investigations involving clinical and laboratory data are warranted.
This study was supported by grants from the Nazarbayev University Faculty Development Research Grant Program FDCRGP 2020-2022 (Funder Project Reference: 240919FD3913).