
Evaluation of a low dose recombinant collagenase and BP protease for clinical islet transplantation
Doug O'Gorman1, Tatsuya Kin1, Shawn Rosichuk1, Brad Richer1, Wendy Zhai1, Jennifer Moriarty1, Kyle Park1, Robert C. McCarthy2, Mike L. Green2, Andrew Briete2, Peter A. Senior1,3, James AM Shapiro1,4.
1Clinical Islet Transplant Program, Alberta Health Services, Edmonton, AB, Canada; 2Vitacyte LLC, ., Indianapolis, IN, United States; 3Dept of Medicine - Endocrinology Division, University of Alberta, Edmonton, AB, Canada; 4Dept of Surgery, University of Alberta, Edmonton, AB, Canada
Introduction: Enzymatic dissociation of the pancreas is a critical step in effectively and efficiently isolating islet cells from surrounding acinar tissue. The development of GMP grade enzymes with improved purities, reduced degradation and clearly defined specifications has contributed to improved isolation success. Continued optimization of enzyme selection, dose and delivery has the potential of improving clinical outcomes by maximizing the islet yield while improving the recovered islets viability and function. In this study we assessed the clinical islet isolation and transplant outcomes using a recombinant C. histolyticum collagenase blend along with B. polymyxa protease (rColl/BPP) at a defined dose in comparison to the current standard of care protocol.
Methods: All isolations were performed using a standardized protocol for the manufacturing of clinical grade islets. rColl/BPP was administered at a target dose of 16 U/g and 22,000 U/g for collagenase and BP protease respectively (n=49). The intra-processing variables as well as isolation and transplant outcomes, should they have fulfilled the criteria, were compared to our current enzyme strategy utilizing 2 separate GMP grade products with an undefined dose spectrum (Control1 n=109 and Control2 n=229). Islet preparations that proceeded to transplantation were cultured at 22°C until time of the procedure and an aliquot was assessed for dynamic glucose stimulated insulin response (GSIR) using the BioRep perifusion apparatus. Intra-portal transplants were performed by a transhepatic approach under local anesthesia.
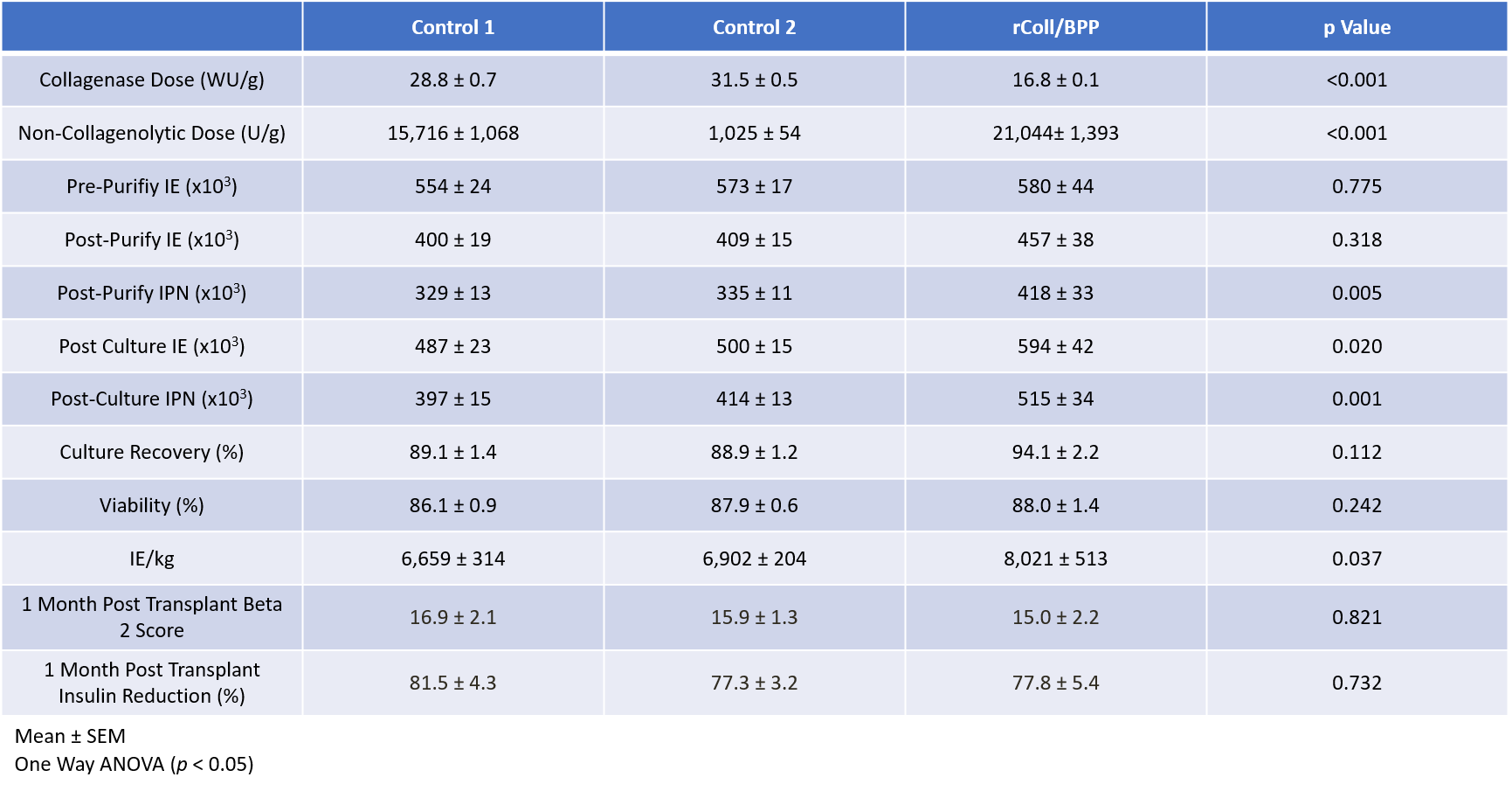
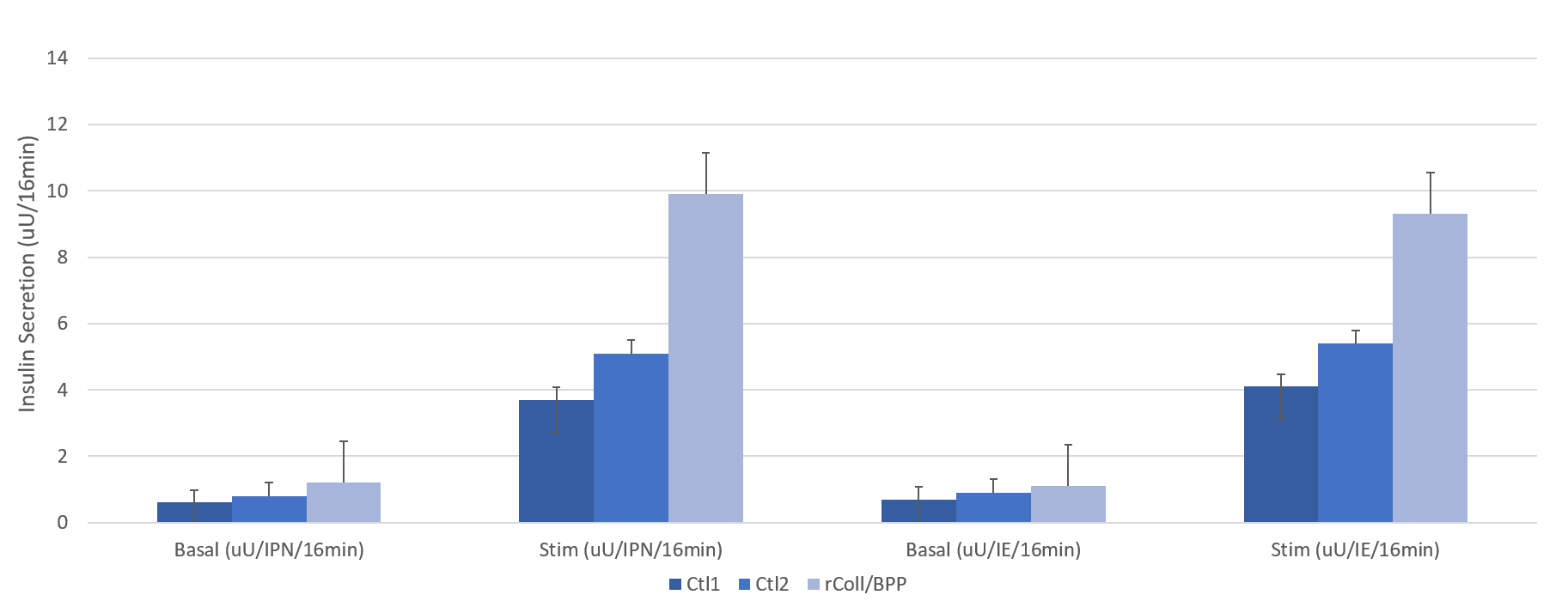
Results: Donor characteristics and digestion profiles among the three groups were similar. Islet yields at the various time points along with transplant outcomes are described in Table 1. A numerically greater islet yield was observed in the rColl/BP at all timepoints and the post culture islet mass was statistically higher. The transplanted Islet Mass was also significantly higher when using rColl/BPP enzyme. Negative correlations were observed between collagenase dose and IE and Islet Particle Number at all points of islet quantification. When assessing GSIR the rColl/BPP group had a significantly higher response to high glucose compared the control groups (Figure 1). Clinical Islet transplant outcomes were similar with all groups achieving similar BETA-2 scores and reductions in insulin requirements at 1 month post-transplant.
Conclusions: Our study suggests that utilizing rColl/BPP enzyme at the prescribed dose can effectively and consistently isolate clinical grade islets and may improve islet recovery. Additionally, this enzyme preserves glucose responsiveness of the islets however this requires additional investigation to the mechanism as it is not clear whether it is the result of the recombinant collagenase, the BP protease, the dose, or a combination of all three factors. Additional studies are underway to investigate this in more depth.