Gene expression and function in pig islets from one-day-old pigs
Kieran Purich1,2, Jim Wickware1,2, Thomas Williams1,2, Adnan Black1,2, Gina Rayat1,2,3,4.
1Department of Surgery, University of Alberta, Edmonton, AB, Canada; 2Alberta Diabetes Institute, University of Alberta, Edmonton, AB, Canada; 3Alberta Transplant Institute, University of Alberta, Edmonton, AB, Canada; 4Ray Rajotte Surgical-Medical Research Institute, University of Alberta, Edmonton, AB, Canada
Introduction: Islet transplantation is a budding alternative treatment for type 1 diabetics. A potential solution to the limited number of human pancreas donors is pig islet xenotransplantation. Limited study has been performed on pig islet cell signaling pathways, which may differ from the mechanisms seen in humans. We have identified molecules involved in the insulin secretion pathway, including the glucose transporter GLUT2, GTPase RAC1, which is involved in F-actin remodeling in islet cells, and SNAP25, a protein involved in insulin release. We evaluated these molecules across different days of culture in neonatal pig islets. We also quantified the gene expression of common cadherins associated with cell-cell adhesion and islet function as determined by insulin secretion.
Method: Islets from 1-day-old pigs (n=3) were isolated and samples collected on days 0, 3, 5 and 7 of culture to quantify GLUT2, SNAP25, RAC1, E-, N- and VE-cadherin gene expression by qRT-PCR. Immunofluorescence staining was performed to localize protein expression. Static glucose stimulation insulin secretion assays (GSIS) and ELISA were used to quantify the ability of these islets to secrete insulin under conditions containing low glucose, high glucose and high glucose with KCl. Kruskal-Wallis testing was completed with GraphPad Prism software.
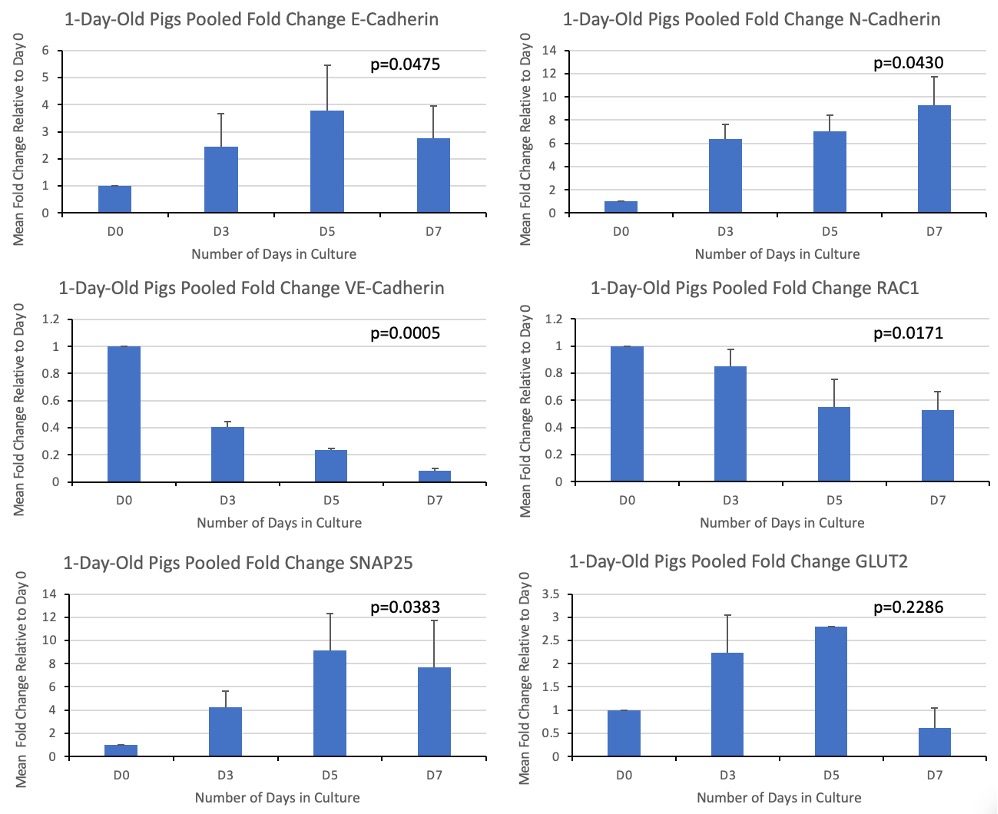
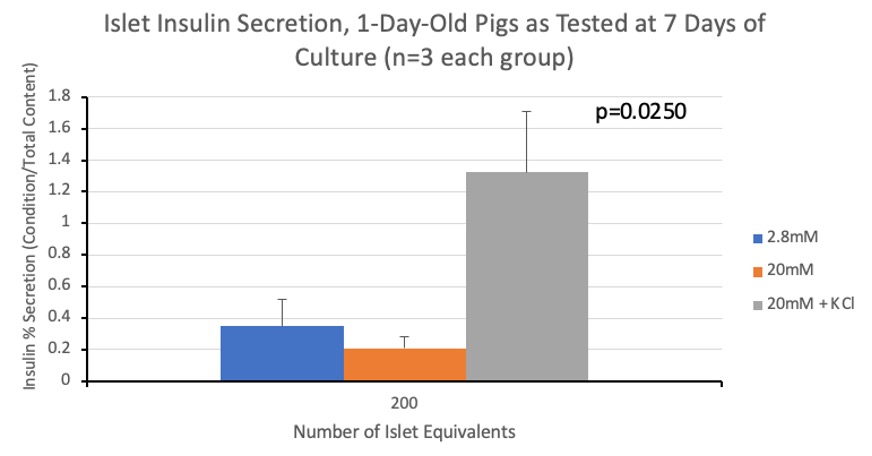
Results: Qualitatively, the islets developed visible membranes and had fewer contaminating exocrine cells as they moved from 0 to 7 days in culture. E-cadherin, SNAP25 and GLUT2 all peaked on day 5 of culture, correlating with when islets formed a distinct spherical structure in-vitro. N-cadherin consistently rose, peaking at day 7 of culture. VE-cadherin was highest at day 0 and decreased with a linear trend. RAC1 gene expression decreased from day 0, remaining stable between days 5 and 7. P-values are indicated in Figure 1. Preliminary immunofluorescence staining showed co-localization of SNAP25 and insulin at all-time points. The amount of insulin secreted in low vs high glucose settings was similar without KCl, and as expected, the amount of insulin secreted when islets were cultured in high glucose with KCl was greater. Kruskal-Wallis testing demonstrated significant differences amongst GSIS groups (Figure 2).
Discussion/Conclusion: Our data demonstrates variation in the gene expression of our molecules of interest across 7 days of culture. Our immunofluorescence results demonstrate that SNAP25 colocalized with insulin, suggesting their proximity in islet cells. Our results also demonstrate the limited ability of glucose to stimulate islets from 1-day-old pigs at 7 days of culture. This is different than results seen previously in our lab suggesting islets from 7-day-old pigs at 7 days of culture secrete greater amounts of insulin when exposed to high glucose conditions. These findings may be important for the development of pig islets in vitro and warrant further investigation.
Walter H. John's Graduate Fellowship (University of Alberta). Royal College of Physicians and Surgeons of Canada (RCPSC) - Clinician Investigator Program. Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant. Canadian Institutes of Health Research (CIHR) - Canadian Graduate Scholarships - Master's Program. Alberta Graduate Excellence Scholarship (AGES).