Establishing the controlled delivery of VEGF using a hydrogel loaded soft robotic drug delivery system with the aim to prevascularise implant site for islet transplantation
Eimear Wallace1,4, Tapas Mitra1,3, Lucien H. J. Schreiber1,4, Giulia Lattinizi1, Gabriella Bellavia4, Garry P. Duffy1,2,3.
1Anatomy and Regenerative Medicine Institute (REMEDI), School of Medicine, National University of Ireland Galway, Galway, Ireland; 2Advanced Materials and BioEngineering Research Centre (AMBER), Trinity College Dublin, Dublin, Ireland; 3CÚRAM, Centre for Research in Medical Devices, National University of Ireland Galway, Galway, Ireland; 4Aurum Laboratories, Explora-Biotech, Rome, Italy
Duffy Lab Group, National University of Ireland Galway.
Introduction: Over 60% of islets transplanted in macrodevices are lost immediately post transplantation due to hypoxia from inadequate early vascularisation [1][2][3]. Prevascularisation of the implant site by spatiotemporally delivering vascular endothelial growth factor (VEGF), the most prominent angiogenic growth factor [4], is a potential solution [5]. VEGF has a half-life of only 50 mins at body temperature [6][7] meaning multiple, large doses are required if delivered systemically causing the formation of unstable, leaky blood vessels [7][8] and adverse off-target effects [9]. Current research focuses on encapsulating VEGF in polymer systems via electrostatic interactions, which minimise the risk of protein denaturation [10], but can require a mechanical stimulus to facilitate its release. Mooney et al. increased VEGF release two-fold from alginate hydrogels with mechanical signalling compared to static hydrogel in vitro and when implanted in diabetic mice after femoral artery ligation, collateral vessel formation increased in mechanically stimulated implant sites [11]. Soft robotics utilise materials with similar elastic modulus to soft tissue and can be safely implanted to facilitate mechanical signalling [12]. Dolan et al. showed that modified biomechanics can increase angiogenesis in rats using soft robotic technologies [13]. We aim to release a predetermined amount of electrostatically interacted VEGF from a hydrogel loaded Soft Robotic Drug Delivery (SRDD) system to stimulate angiogenesis in the underlying tissue.
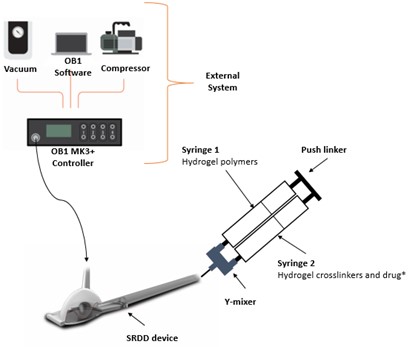
Methods: In vitro release studies were performed to optimise an actuation regime for controlled release of the model drug, Fluorescein isothiocyanate–Diethylaminoethyl–Dextran (Dextran) (same charge and molecular weight as VEGF), from a hydrogel loaded SRDD device. The SRDD device was submerged in release media, connected to the external system (Figure 1), actuated using a customisable actuation regime (MatLab®), and concentration of released Dextran determined using absorbance measurements. The actuation regime was modified until the optimal pressure, ramp, and cycle number were selected to facilitate controlled release of Dextran.
Results: In the absence of mechanical stimulation, the passive release of Dextran was minimal (<0%). Increasing mechanical stimulation from 0-12 psi increased the release rate of Dextran during pressure optimisation (0 vs 0.15%, p=0.45). Ramp times of 5-30 secs further increased Dextran release (0 vs 2.38%, p=0.06), with ramp of 5-sec showing constant release at each time point. Using 10 psi and 5-sec ramp, cycle numbers were varied from 2-10 with 5 and 10 cycles releasing the greatest amount of Dextran (53.79 vs 68.97%, p<0.005, Figure 2).
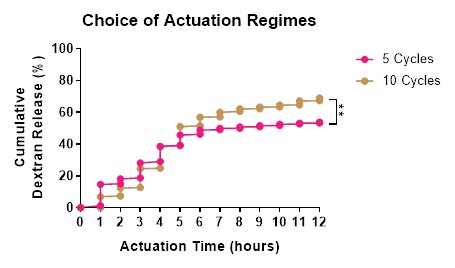
Conclusion: Actuation of a hydrogel loaded SRDD device is modifiable to facilitate the controlled release of Dextran, which is important for our intended use of releasing VEGF spatiotemporally to prevascularise an implantation site for islet transplantation.
DELIVER project that received funding from the European Union’s Horizon 2020 Marie Sklodowska-Curie Actions programme under grant agreement number 812865.
[1] M. McCall and A. M. James Shapiro, “Update on islet transplantation,” Cold Spring Harb. Perspect. Med., vol. 2, no. 7, 2012, doi: 10.1101/cshperspect.a007823
[2] J. A. Emamaullee and A. M. James Shapiro, “Factors Influencing the Loss of-Cell Mass in Islet Transplantation,” Cell Transplant., vol. 16, pp. 1–8, 2007, Accessed: Apr. 14, 2020. [Online]. Available: www.cognizantcommunication.com
[3] M. Khosravi-Maharlooei et al., “Therapy of endocrine disease: Islet transplantation for type 1 diabetes: So close and yet so far away,” European Journal of Endocrinology, vol. 173, no. 5. BioScientifica Ltd., pp. R165–R183, Nov. 01, 2015, doi: 10.1530/EJE-15-0094
[4] K. Skrzypek, M. G. Nibbelink, L. P. Karbaat, M. Karperien, A. van Apeldoorn, and D. Stamatialis, “An important step towards a prevascularized islet macroencapsulation device—effect of micropatterned membranes on development of endothelial cell network,” J. Mater. Sci. Mater. Med., vol. 29, no. 7, Jul. 2018, doi: 10.1007/s10856-018-6102-0
[5] M. W. Laschke and M. D. Menger, “Prevascularization in tissue engineering: Current concepts and future directions,” Biotechnology Advances, vol. 34, no. 2. Elsevier Inc., pp. 112–121, Mar. 01, 2016, doi: 10.1016/j.biotechadv.2015.12.004
[6] Z. Wang, Z. Wang, W. W. Lu, W. Zhen, D. Yang, and S. Peng, “Novel biomaterial strategies for controlled growth factor delivery for biomedical applications,” 2017, doi: 10.1038/am.2017.171
[7] J. D. Weaver et al., “Vasculogenic hydrogel enhances islet survival, engraftment, and function in leading extrahepatic sites,” Sci. Adv., vol. 3, no. 6, Jun. 2017, doi: 10.1126/sciadv.1700184
[8] N. Ferrara, H. P. Gerber, and J. LeCouter, “The biology of VEGF and its receptors,” Nature Medicine, vol. 9, no. 6. Nature Publishing Group, pp. 669–676, Jun. 01, 2003, doi: 10.1038/nm0603-669
[9] K. Lee, E. A. Silva, and D. J. Mooney, “Growth factor delivery-based tissue engineering: General approaches and a review of recent developments,” Journal of the Royal Society Interface, vol. 8, no. 55. Royal Society, pp. 153–170, Feb. 06, 2011, doi: 10.1098/rsif.2010.0223
[10] F. Gu, B. Amsden, and R. Neufeld, “Sustained delivery of vascular endothelial growth factor with alginate beads,” J. Control. Release, vol. 96, no. 3, pp. 463–472, May 2004, doi: 10.1016/j.jconrel.2004.02.021
[11] K. Y. Lee, M. C. Peters, K. W. Anderson, and D. J. Mooney, “Controlled growth factor release from synthetic extracellular matrices,” Nature, vol. 408, no. 6815, pp. 998–1000, 2000, doi: 10.1038/35050141
[12] P. Polygerinos et al., “Soft Robotics: Review of Fluid-Driven Intrinsically Soft Devices; Manufacturing, Sensing, Control, and Applications in Human-Robot Interaction,” Advanced Engineering Materials, vol. 19, no. 12. Wiley-VCH Verlag, Dec. 01, 2017, doi: 10.1002/adem.201700016
[13] E. B. Dolan et al., “An actuatable soft reservoir modulates host foreign body response,” Sci. Robot., vol. 4, no. 33, p. eaax7043, 2019, doi: 10.1126/scirobotics.aax7043