
Analysis of autoimmune re-activation after Covid-19 mRNA vaccination in pancreas transplant recipients
Caterina Di Bella1, Cristina Crepaldi2, Monica Vedovato2, Marianna Di Bello1, Georgie Innico3, Rita Andreuzza2, Angelo Avogaro2, Paolo Rigotti1, Gian Paolo Fadini2, Lucrezia Furian1.
1Kidney and Pancreas Transplant Unit, University of Padua, Padua, Italy; 2Department of Medicine, University of Padua, Padua, Italy; 3Nephrology, Dialysis and Transplantation Unit, University of Padua, Padua, Italy
Introduction: Type 1 diabetes (T1D) results from the autoimmune destruction of insulin-producing pancreatic β cells. The autoimmune process acts insidiously for many years and clinical symptoms appear when up to 80% of β cells have been destroyed. As a result, islet autoantibodies titre decreases over time. After pancreas transplantation (PT), lifelong immunosuppression (IS) helps in keeping autoantibodies titre low and inhibiting the immunological relapse.
Transplanted patients should be prioritized for COVID 19- vaccination but vaccine-associated autoimmunity is a concern. The fear of triggering a cross-reactivity responsible for the recurrence of immune-mediated disorders after COVID-19 vaccination might be discouraging.
Aim of the study is to evaluate the relapse of immune-mediated process induced by COVID-19 mRNA vaccine, in patients with T1D and PT.
Methods: We collected data on 25 patients with SPK or PTA who completed vaccination with two doses of COVID-19 mRNA vaccine. Serum markers of aberrant activation of the innate immune system, inflammation and hemolysis (C3 and C4 factor, haptoglobin, CRP, fibrinogen, LDH, d-dimer, blood count), titres of islet autoantibodies (anti-GAD, anti-IA-2, and anti-ICA), pancreatic enzymes, glycated hemoglobin (HbA1c) and C-peptide were measured to identify immunological activation and graft function. The analyses were performed before starting vaccination and within one month after the second dose of COVID-19 mRNA vaccine.
Results: Overall, we did not identify major signs of inflammation. Seven patients showed elevated D-dimer after vaccination. In 12 patients, there was complement factor 3 consumption without signs of inflammation. Mean serum CRP was 4.05±2.12 mg/L. There were no significant changes in blood count, haptoglobin, and LDH, ruling out intravascular hemolysis. The comparison of islet autoantibodies titre before and after vaccination did not reveal any consistent autoimmune re-activation (Table 1).
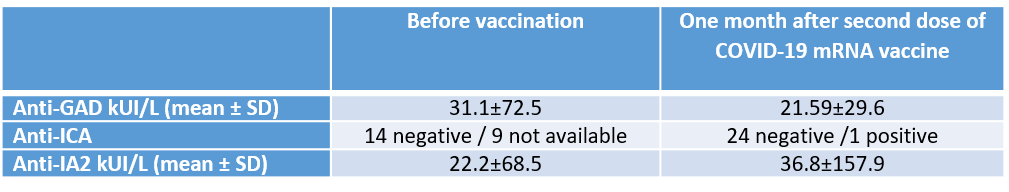
No alteration in pancreatic enzymes was found. One patient who requires low-dose insulin had positive ICA and high level of IA2. Graft function was stable in all the other patients with a mean serum C-peptide of 3.03±1.04 ug/L and HbA1c of 35.6±4.7 mmol/mol (%), after completing vaccination.
Discussion: Viruses are involved in the pathogenesis of autoimmune diseases while mRNA vaccines can trigger immunological events. Our preliminary data do not show consistent recurrence of immune-mediated processes in patients with PT due to T1D, who are predisposed to autoimmunity but are on chronic IS.
IS may mitigate vaccine immunogenicity, as demonstrated by the low levels of detectable antibodies against SARS-CoV-2 Spike protein after receiving vaccination, described in the current literature.
At the present, the evidence-based benefits of COVID-19 vaccination in PT patients outweigh the risk associated with a hypothetical autoimmune re-activation due to molecular mimicry between the spike protein of SARS-CoV-2 and self-antigens, such as β cells islets.