
Predictive Value of C-peptide Measures for Clinical Outcomes of Islet Transplantation in Type 1 Diabetes: a Report from the Collaborative Islet Transplant Registry (CITR)
David Baidal1, Michael Rickels2, Cassandra Ballou3, Elizabeth Payne3, Franca Barton3, Rodolfo Alejandro1.
1Medicine and the Diabetes Research Institute, University of Miami, Miami, FL, United States; 2Medicine, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, United States; 3Collaborative Islet Transplant Registry Coordinating Center, The EMMES Company, LLC, Rockville, MD, United States
CITR Investigators.
Introduction: Long-standing type 1 diabetes (T1D) can lead to impaired awareness of hypoglycemia and high-risk for severe hypoglycemic episodes (SHE). Islet transplantation (ITx) can restore endogenous insulin production, ameliorate glucose control, and abolish SHE. In the DCCT, a stimulated C-peptide >0.2 nmol/L was associated with lower HbA1c, reduced insulin requirements, and decreased prevalence of SHE. We set forth to determine what measures of C-peptide and levels are associated with achievement of ITx positive outcomes in T1D.
Methods: Collaborative Islet Transplant Registry (CITR) Islet-Alone recipients with pre-transplant C-peptide <0.1 nmol/L and mean follow up of 4.6±1.1 years were included in the analyses (n=677). Post-transplant metabolic measures obtained from arginine stimulation test, IVGTT, OGTT, glucagon stimulation test and mixed meal stimulation test (MMTT) were evaluated. Predictive value of these measures for 7 primary outcomes of clinical ITx (i.e., absence of SHEs (ASHE); HbA1c<7.0%; HbA1c <7.0% & ASHE; HbA1c≤6.5; HbA1c≤6.5% & ASHE; insulin independence; and ASHE, HbA1c≤6.5%, and insulin independence) was assessed by area under the curve of receiver-operator characteristics curves (ROC-AUC). Measures with the highest ROC-AUC were then selected for determination of optimal cut points by ITx outcome.
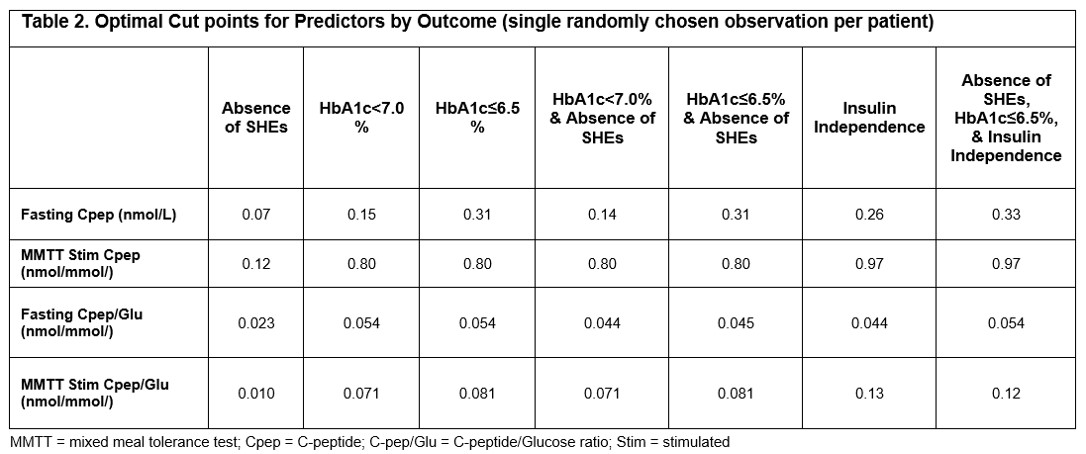
Results: Fasting C-peptide, MMTT stimulated C-peptide, fasting C-peptide to Glucose (Cpep/Glu) ratio, and MMTT stimulated Cpep/Glu ratio showed the highest ROC-AUCs among all measures evaluated (Table 1). Fasting C-peptide was highly predictive for ASHE (ROC-AUC = 0.9228; optimal cut point = 0.07 nmol/L), and the composite outcome of ASHE, HbA1c≤6.5%, and insulin independence (ROC-AUC = 0.8456; optimal cut point = 0.33 nmol/L) (Table 2). However, MMTT stimulated Cpep/Glu ratio outperformed both fasting and stimulated C-peptide for all outcomes except ASHE alone. Optimal cut point for the ideal composite outcome of ASHE, HbA1c≤6.5%, and insulin independence was 0.12 nmol/mmol for the MMTT stimulated Cpep/Glu ratio, and 0.97 nmol/L for MMTT stimulated C-peptide (Table 2).
Discussion/Conclusion: Fasting C-peptide reliably predicts rates of ITx primary endpoints and is similar to other C-peptide measures at predicting ASHE. Although marginal endogenous fasting C-peptide is sufficient to eliminate SHEs, higher levels are required to achieve optimal ITx outcomes. The MMTT stimulated Cpep/Glu ratio provides better predictiveness for composite ITX outcomes, including insulin independence. In the absence of a MMTT, a fasting C-peptide ≥0.33 nmol/L is a reassuring measure of optimal metabolic control in ITx T1D recipients.

The CITR has been funded continuously by grants from the National Institute of Diabetes and Digestive and Kidney Diseases since 2001 (Grants 1UCHDK098086-01, 1UC4DK114839-01) to collect data on the efficacy and safety of clinical islet transplantation from US and collaborating international institutions. The Juvenile Diabetes Research Foundation provided supplemental funding for reimbursement of efforts to report data from the European and Australian sites from 2006-2015. No other sponsors have provided support to the CITR. MRR is supported in part by Public Health Service Research Grant R01 DK 091331-8. We thank all the study participants and staff .