Characterizing the therapeutic potential of human pluripotent stem cell-derived islet-like cells lacking cell-surface HLA expression
Paraish Misra1,2,6, Jong Bok Lee3,4, Li Zhang3,4, David W. Russell5, M. Cristina Nostro1,2.
1McEwen Stem Cell Institute, University Health Network, Toronto, ON, Canada; 2Department of Physiology, University of Toronto, Toronto, ON, Canada; 3Toronto General Hospital Research Institute, University Health Network, Toronto, ON, Canada; 4Department of Immunology, University of Toronto, Toronto, ON, Canada; 5Department of Medicine, University of Washington, Seattle, WA, United States; 6Department of Medicine, University of Toronto, Toronto, ON, Canada
Introduction: While islet or pancreas transplantation remains the optimal way of restoring physiologic blood glucose control in patients with Type 1 diabetes, these therapies are limited by a scarcity of organs available for transplantation and the requirement for immunosuppression to prevent rejection. Recently, effective methods to generate functional islet-like cells (ILCs) from human pluripotent stem cells (hPSCs) have been developed, which opens the possibility for organ replacement without the limitation of scarcity. However, hPSC-derived ILCs would still be expected to be rejected without immunosuppression, which would increase the toxicity associated with this therapy. Because HLA proteins are considered to be primary triggers of allorecognition and rejection, we sought to determine whether hPSCs deficient for cell-surface HLA class I and II expression could differentiate into ILCs less prone to allorejection.
Methods: We differentiated HLA-control and HLA-deficient hPSCs into ILCs using an optimized 23 day differentiation protocol. Cells were characterized for pancreatic islet markers, cell surface HLA antigens, and co-stimulatory ligands by flow cytometry. The allo-immunogenicity of control and HLA-deficient ILCs was determined by their ability to induce proliferation, as assessed by CFSE dilution, in allogeneic CD4+ and CD8+ T cells isolated from healthy donor peripheral blood mononuclear cells.
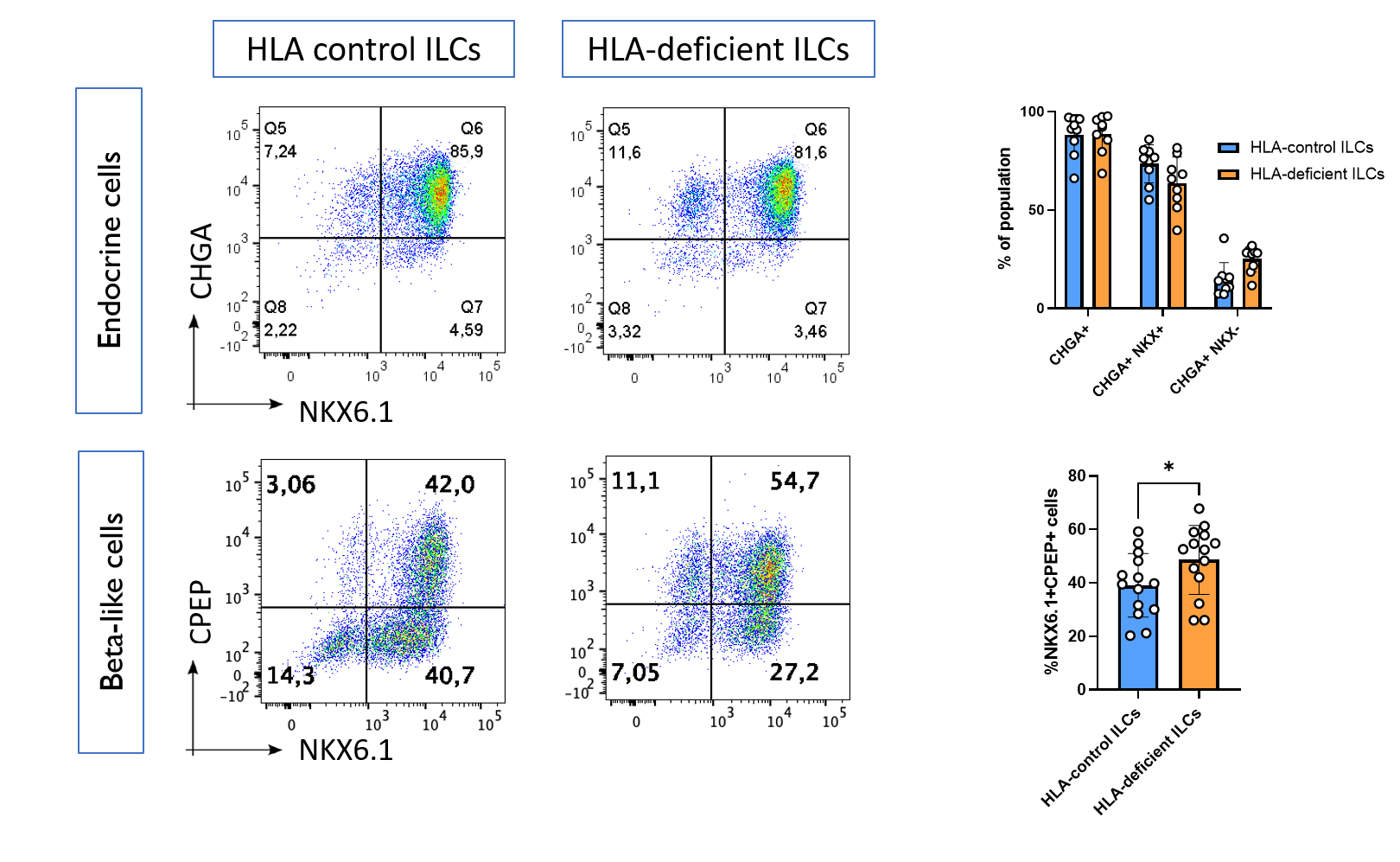
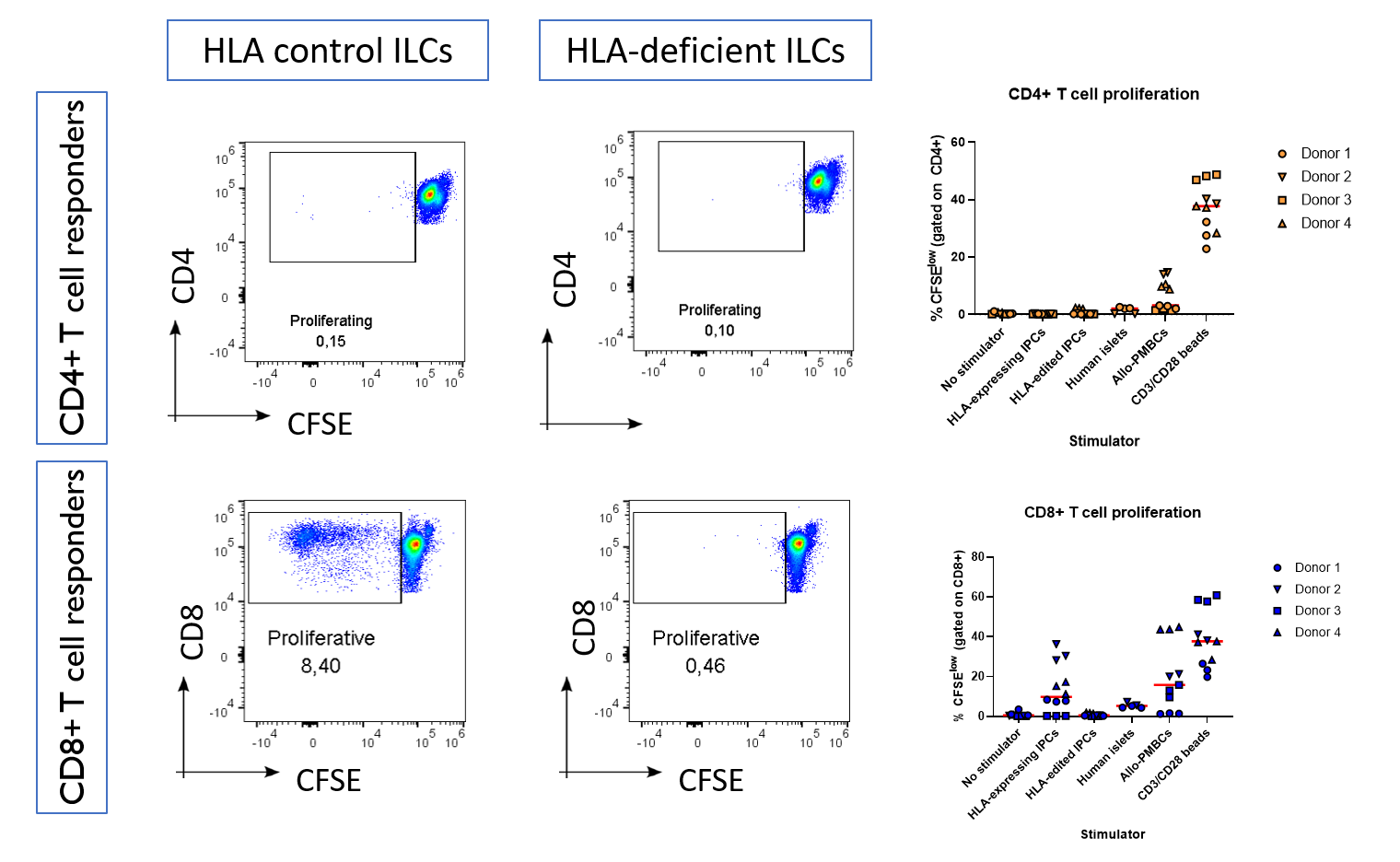
Results: We found that HLA-deficient hPSCs exhibited no impairment in their differentiation into ILCs, with both control and HLA-deficient hPSCs generating populations with >90% expression of the endocrine marker Chromogranin A, as assessed by flow cytometry (Figure 1). Unexpectedly, HLA-deficient hPSCs contained a higher proportion of NKX6.1+CPEP+ beta-like cells compared with control hPSCs (p = 0.03). HLA-control ILCs, HLA-deficient ILCs, and primary human islets had similar cell-surface expression of immunomodulatory ligands, with the notable exception that only HLA-deficient ILCs did not express any HLA class I. Upon co-culture with primary allogeneic T cells, control ILCs induced robust activation of CD8+ T cells isolated from 3 out of 4 healthy donors, while HLA-deficient ILCs were not able to induce activation in any of the 4 donors (Figure 2).
Discussion/Conclusion: We have found that HLA-deficient hPSCs exhibit no impairment in the ability to differentiate into ILC, and that HLA-deficient ILCs have advantageous immunologic properties. This suggests that patients treated with these cells may not require as much immunosuppression to avoid allorejection. HLA-deficient ILCs may thus be superior candidates for clinical translation for the treatment of type 1 diabetes.