An initial analysis of the baseline levels of dd-cfDNA after pancreas transplantation: a prospective study from high-volume centers in the United States
Ashley Yoo1, Ian Qian1, Alexandria Riedel1, Amanda Bartosic1, Rudi Soltani2, Ojel Nduanya3, Marziyeh Omidi3, Jon S. Odorico2, Matthew Cooper3, Jonathan S. Bromberg1, Joseph R. Scalea1.
1Department of Surgery, Division of Transplant Surgery, University of Maryland School of Medicine, Baltimore, MD, United States; 2Department of Surgery, Division of Transplantation, University of Wisconsin School of Medicine and Public Health, Madison, WI, United States; 3Department of Surgery, Division of Kidney and Pancreas Transplantation, Georgetown University School of Medicine, Washington, DC, United States
Introduction: Donor-derived cell-free DNA (dd-cfDNA) testing, which has been validated for monitoring kidney transplant (KTx) rejection, may provide an alternative to pancreas biopsy that prevents morbidity and graft loss. We determined, for the first time, dd-cfDNA levels in pancreas transplant (PTx) recipients with and without rejection.
Methods: From N=101 participants, n=161 dd-cfDNA blood samples up to 50 months post-transplant were collected.
Results: 78 (77%) participants received SPK, 21 (21%) received PTA, 1 (1%) received PAK, and 1 (1%) received KTA. Mean, median, and mode of dd-cfDNA levels in all participants are 0.61±0.98%, 0.28%, and 0.20%. Mean baseline dd-cfDNA level of participants without rejection was 0.46±0.70% [N=96 (95%), n=128 (80%)]. Mean baseline dd-cfDNA levels peaked at 1.45±1.54% within one-month post-transplantation. Compared to baseline, mean dd-cfDNA levels of participants with any rejection were 1.22±0.98% [N=6 (6%), n=33 (20%), p=0.02]. For those with KTx ACR, mean dd-cfDNA levels was 1.54±1.94% [N=2 (2%), n=12 (7%), p=0.11]. For those with PTx rejections overall, ACR, and AMR, mean dd-cfDNA levels were 1.23±1.58% [N=4 (4%), n =24 (15%), p=0.042], 1.51±1.81% [N=3 (3%), n=17 (11%), p=0.02], and 0.54±0.30% [N=1 (1%), n=7 (4%), p=0.49].
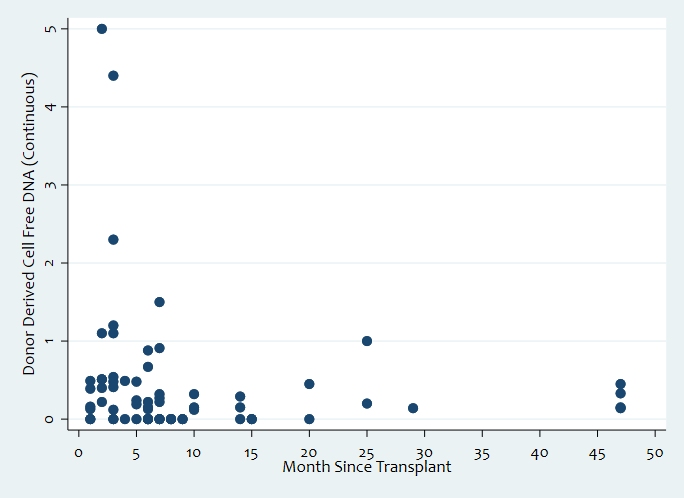
Discussion: The mean baseline dd-cfDNA level for PTx recipients in this study is <1.0%, which is consistent with the KTx rejection threshold dd-cfDNA value in KTx recipients of >1.0% in existing literature. However, the natural course of baseline dd-cfDNA seems to transiently elevate >1.0% at one-month post-transplant. This study shows that dd-cfDNA elevation also correlates with pancreas rejection. The mean dd-cfDNA level in PTx rejections overall and PTx ACR specifically are >1.0% and significantly different from baseline levels. In PTx patients, dd-cfDNA is a promising biomarker for PTx rejection. Accruing new and following existing participants will confirm these findings and help define a rejection threshold.
Funding support from CareDx for this study.