
Long-Term Preservation of Isolated Human, Mouse, Porcine Islets and Human Stem Cell Derived Beta Cells (HUES-8 Cell Lines) Using a High Throughput Vitrification-Rewarming Modified Cryomesh Technique to Successfully Cure Diabetes in a Mouse with Transplantation
Joseph Sushil Rao1,2,6, Li Zhan3,6, Diane Tobolt1,6, Nikhil Sethia3,6, Michael Slama5, Zonghu Han3,6, Peterson Quinn5, Cari Dutcher3, John Bischof3,4,6, Erik B. Finger1,6.
1Solid Organ Transplantation, University of Minnesota, Minneapolis, MN, United States; 2Schulze Diabetes Institute, University of Minnesota, Minneapolis, MN, United States; 3Mechanical Engineering, University of Minnesota, Minneapolis, MN, United States; 4Institute for Engineering in Medicine, University of Minnesota, Minneapolis, MN, United States; 5Center for Regenerative Medicine, Mayo Clinic, Rochester, MN, United States; 6ATP-Bio NSF ERC, University of Minnesota, Minneapolis, MN, United States
Introduction: Islet transplantation has the potential to cure diabetes & a major barrier is the lack of high-quality islet equivalence when & where they are needed, while single donor infusions are often insufficient to render an allo-recipient insulin independent. We propose to utilize vitrification at ultralow temperature (<-150°C) in an ice-free glassy state as an alternative to conventional cryopreservation to achieve a high viability and functional islet pool for long term storage/banking of islets prior to transplantation.
Methods: Mouse islets were isolated from C57BL/6 retired breeders, cultured for 12h in 5% CO2 S3 islet culture media. Cryoprotective Agent (CPA) optimization was performed & shrink-swell parameters measured using microfluidic platforms. Islets were loaded & unloaded with CPA (22% DMSO & 22% Ethylene glycol), vitrified-rewarmed (VR) & unloaded using the modified cryomesh (Nylon – 40µm pore size). Optimization of our protocol was performed at 0-, 3- & 24h after VR. Viability, ATP content, mitochondrial activation, mitochondrial stress/ Oxygen Consumption Rate (OCR), Glucose Stimulated Insulin Secretion (GSIS) were in-vitro assays performed. Brightfield & Transmission Electron Microscopy (TEM) images & Single-cell RNA sequencing was performed. HUES-8 Human Stem Cell Derived Beta Cells (HSc), isolated porcine & human islets were also assayed. Syngeneic B6-B6 islet transplants were performed under kidney capsule. HSc & Pig islet xenotransplants were performed into Nod-Scid-Gamma mice. Islets in-vivo were subjected to glucose stimulation using an intra-peritoneal approach. Nephrectomies were performed on defined post-operative days & imaged using fluorescence antibodies for insulin & glucagon & Blood Glucose (BG) monitoring was continued for the next five days.
Results: Following rewarming, three-hour recovery in a dynamic spinner flask with culture media at 5% CO2 was essential. Viability of 91% was achieved with VR, compared to 59% using conventional cryopreservation methods. ATP content, mitochondrial reactivation, OCR, GSIS demonstrated significant difference compared to conventional cryopreservation in all 4 species. Brightfield and TEM imaging show well preserved cellular architecture. 9/10 VR transplants (250 islets) normalized BG in 48h with tight control of BG thereafter for >100 days with acceptable responses to glucose stress tests, while insulin production of pig & HSc VR xenografts were comparable to control xenotransplants.
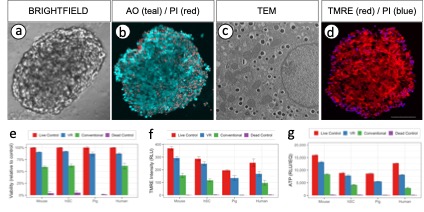
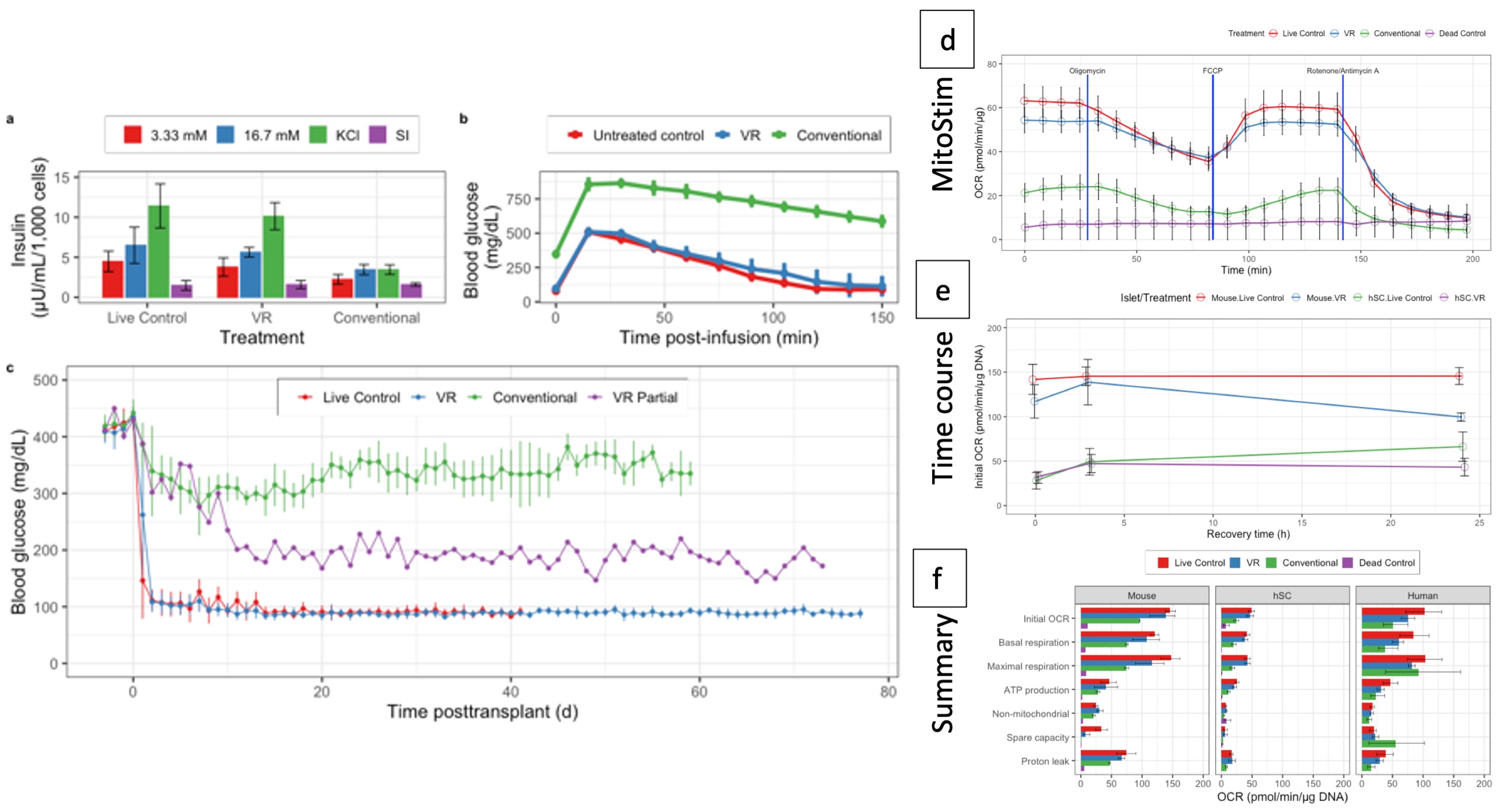
Conclusion: Our optimized CPA loading/unloading protocol, followed by rapid heating – cooling with modified cryomesh VR, enables islet banking with high viability and function for transplantation and cure of diabetes.