
The optimal cell packing density for macroencapsulating islet delivery devices
Rick de Vries1, Mojtaba Barzegari2, Aurélie Carlier1, Liesbeth Geris2, Aart van Apeldoorn1.
1Cell Biology-Inspired Tissue Regeneration, MERLN institute, Maastricht, Netherlands; 2Division of Skeletal Tissue Engineering, Prometheus, Leuven, Belgium
Introduction: Macroencapsulation transplantation devices focused on offering alternative transplantation sites for islets remain an interesting approach. Our group focusses on an ‘open’ macroporous islet delivery device that aims to improve islet revascularization1. A second generation of the device has now been developed using a clinically-approved biomaterial. Device dimensions with the current implant design lead to relatively large clinically-relevant sized devices which are difficult to implant. Hence there is a need to increase the packing density of islets within the medical device, without severely affecting islet viability and functionality.
Method: A second generation of porous, microwell-array islet delivery devices were manufactured in a standardized fashion. A software application was created to upscale the delivery device from mouse-sized towards human-sized implants. A computational islet oxygen consumption model was developed which simulates the impact of islet size and device packing density on its local oxygen levels. Local oxygen levels were also measured in vitro through different oxygen imaging strategies, and used to verify the computational model. Once verified, the model was extrapolated to simulate different upscaling strategies aiming to downsize the final implant dimensions, such as overfilling of microwells or stacking of multiple layers of devices. Finally, this data is used to design islet delivery devices with an optimal packing density.
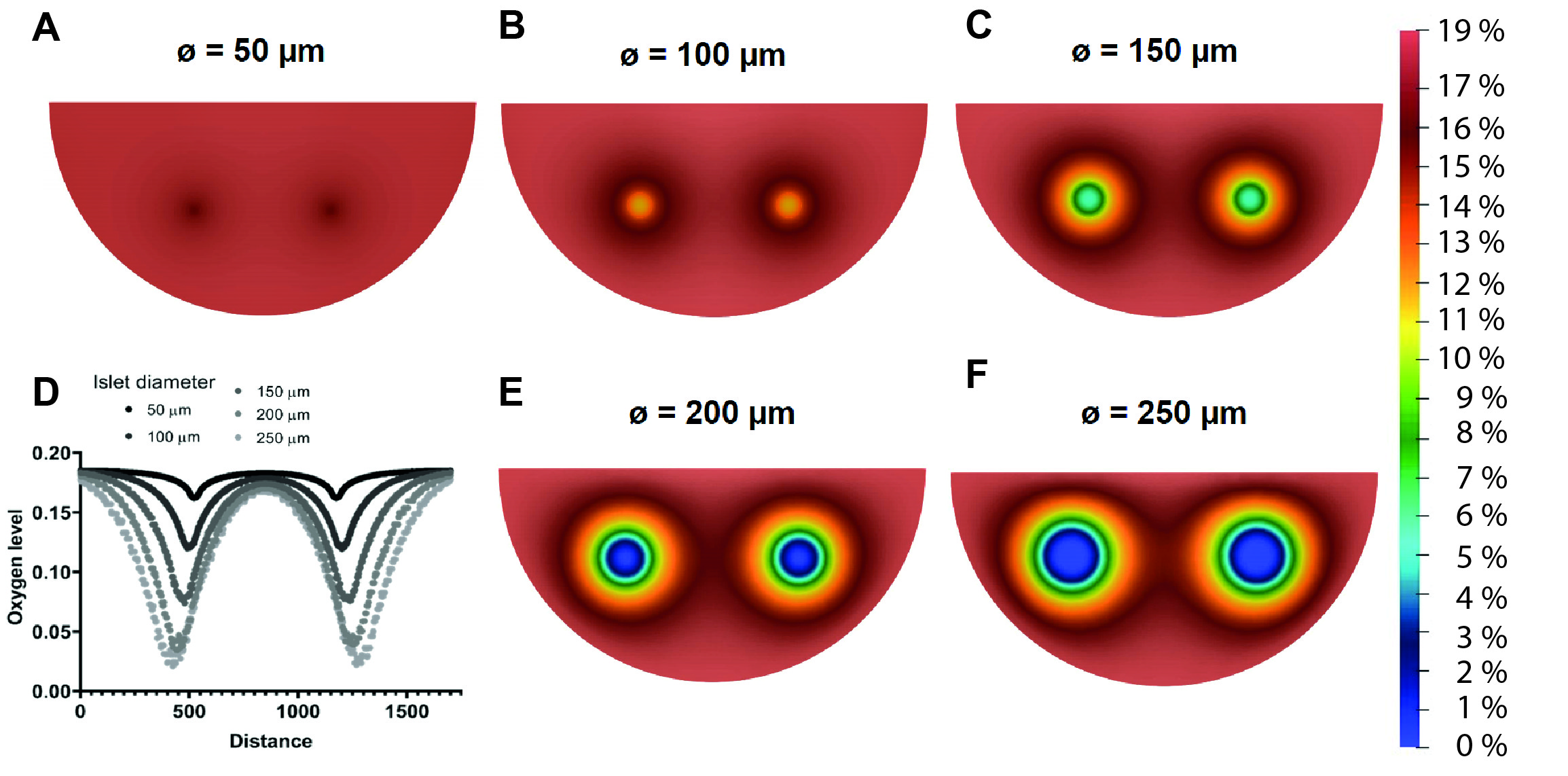
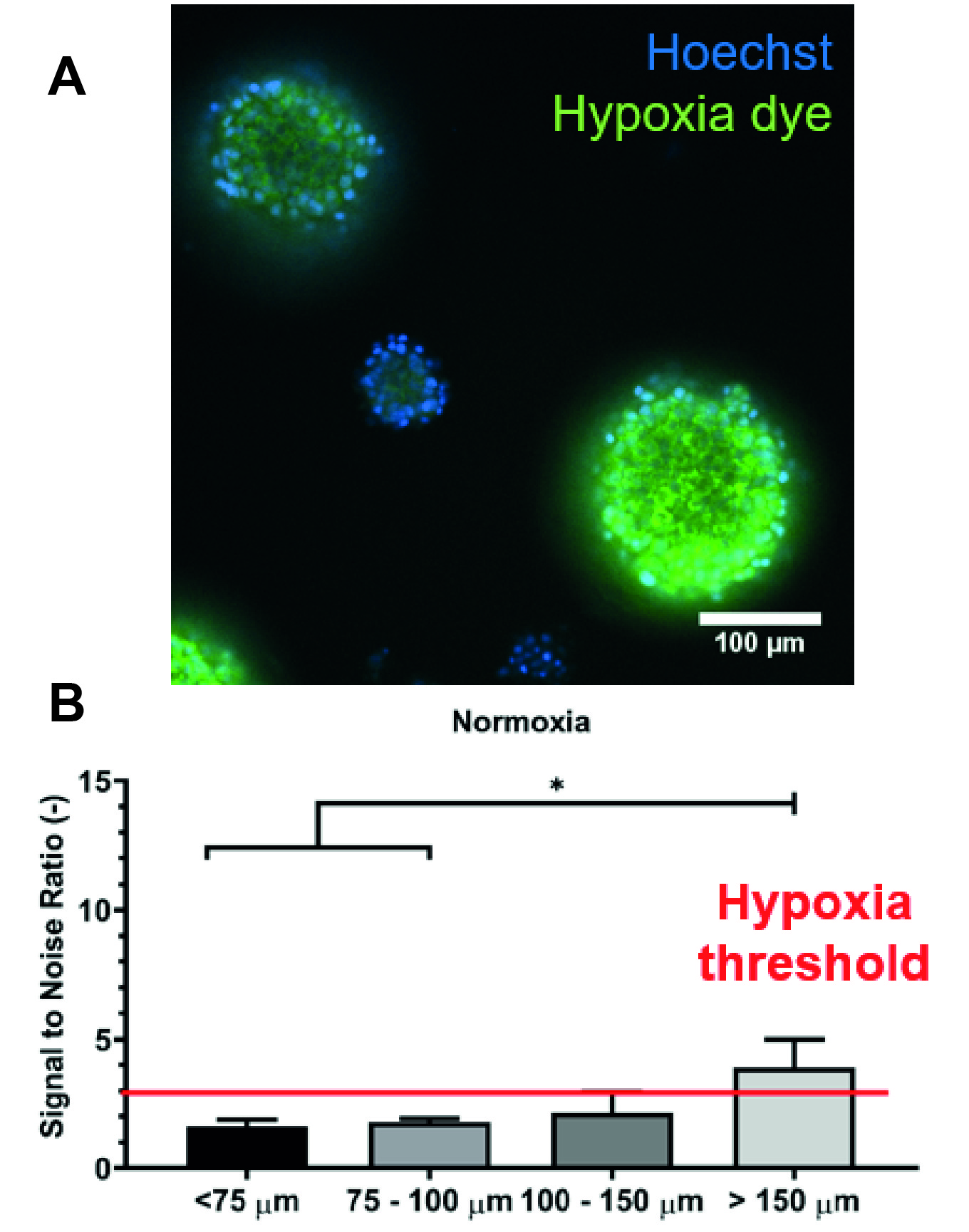
Results: Oxygen imaging was used to measure oxygen kinetics in between islets during normoxia (21% O2) and hypoxia (<5% O2). Most notably, islets with a diameter >150 um showed hypoxic cores even when cultured in normoxia. Local oxygen levels were affected when islets were within 500 μm of one another, even leading to anoxic events if the distance was smaller than 300 μm during hypoxia. In addition, overseeding of microwells with small islets does not lead to hypoxia, while islets with a diameter > 150 um cannot be co-cultured into one microwell without causing severe hypoxia. A software application was developed that allows determination of patient-specific implant dimensions and aids physicians to select which size off-the-shelf implant is most suitable for their patient.
Discussion: This work highlights the importance of islet diameter on islet survival and functionality. The microwells within the macroencapsulation device have a clear benefit, distancing islets from one another and preventing aggregation. Moreover, there is a fine tradeoff between a high packing density and minimal implant dimensions, and a relatively low packing density leading to implant dimensions that are unfeasible in the clinic. The in silico local oxygen level predictions seem to overlap with in vitro oxygen imaging results. The model was therefore extrapolated, allowing the evaluation of several implant downsizing strategies.
1 Buitinga M, Assen F. Biomaterials. 135:10-22, 2017
This project was funded by the Juvenile Diabetes Research Foundation, grant key 3-SRA-2016-256-S-B.