Immune Response to Vascularizing Subcutaneous Engineered Islet Grafts
Krystal Ortaleza1, Sean M. Kinney1,2, So-Yoon Won1, Ilana Talior-Volodarsky1, Michael Sefton1,2.
1Institute of Biomaterials and Biomedical Engineering, University of Toronto, Toronto, ON, Canada; 2Department of Chemical Engineering and Applied Chemistry , University of Toronto, Toronto, ON, Canada
Introduction: The subcutaneous space is a promising islet transplant site as it has the necessary large transplant volume and largely avoids the blood-mediated inflammatory response, but it requires further vascularization. Moreover, the accessibility of this site allows for monitoring and retrieval of the transplant.
Our lab’s methacrylic acid (MAA)-containing biomaterials induce vascularization, in part by polarizing macrophages to a pro-regenerative “M2” type state. We have shown that rodent islets injected in an MAA-poly(ethylene glycol) (MAA-PEG) hydrogel, survive and engraft in the subcutaneous space of immune compromised SCID/Bg mice. It is expected that the modulation of host macrophages plays an important role in subcutaneous islet engraftment, through an effect on vessel formation and IGF1 signaling, but the extent to which this polarization affects the local immune environment is still unknown. We expect that the unique immune environment of the skin together with MAA-modulated vascularization will affect the efficacy of peri-operative immunosuppressants commonly used for allogeneic transplants. In this work we aim to understand the immune environment of subcutaneously implanted, vascularizing islet allografts to allow for the development of a targeted short-term immune mitigation strategy.
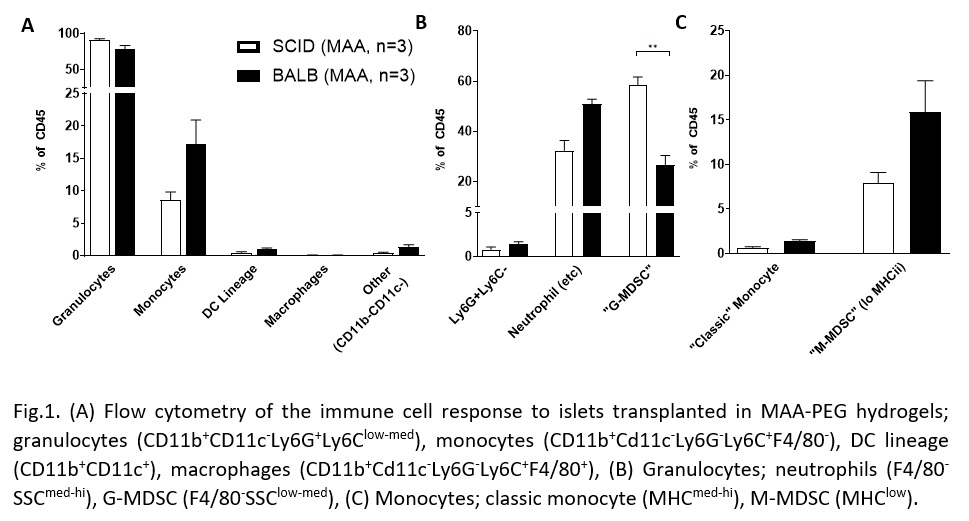
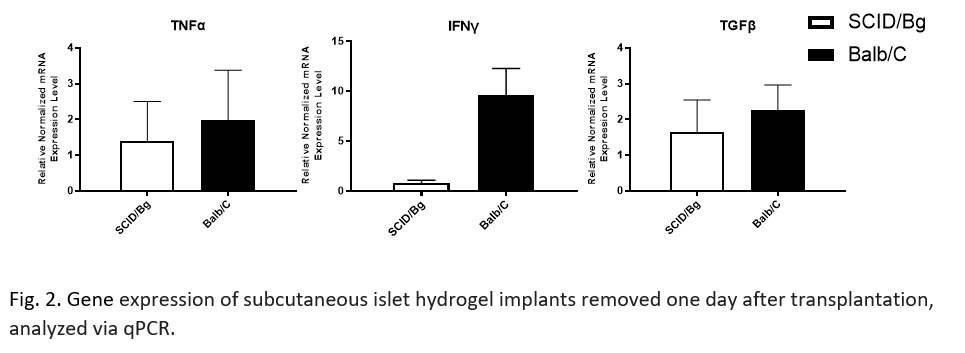
Methods: 200 mouse islet equivalents (IEQ) were isolated from C57Bl/6J mice and injected in 100 µl of MAA-PEG hydrogel into Balb/c or SCID/bg mice. Islet grafts were removed on days 1, 3, and 7 for analysis of the immune environment by flow cytometry or qPCR.
Results: Granulocyte and monocyte recruitment differed between allografts in BALB/c mice and an immunocompromised SCID/bg “immunological control” at day 1. Of note was a significant difference in an apparent granulocyte myeloid derived suppressor cell (MDSC) population (CD11b+CD11c-Ly6G+Ly6Clow-med F4/80-SSClow-med). Differences in IFNy, but not TNFα or TGFβ, expression were also observed.
Discussion/Conclusion: We have identified differences in innate cell recruitment between immune competent and incompetent models, particularly the MDSC populations. MDSCs are key players in allograft acceptance because of their mediation of vascularization-associated inflammation. A better understanding of this difference will help determine if these cells are required for proper islet engraftment in a vascularized subcutaneous site. Characterization of the implant also revealed an upregulation of IFN-γ expression. This upregulation highlights that local macrophages or natural killer cells may have been activated by the graft. Work is ongoing to further explore these results and evaluate the efficacy of common immunosuppressants on the response to a subcutaneous allograft. A greater understanding of the immune response to a vascularizing, subcutaneous islet graft will allow us to better develop strategies for subcutaneous islet transplantation as a regenerative treatment option for T1D.
CFREF/Medicine by Design. Canadian Institutes of Health Research.