
Stimulation of intra-islet endothelial cells to form peri-islet vessels: Bioengineering approaches for improving human islet microcirculation and function
Appakalai Natarajan Balamurugan1, Siddharth Narayanan1, Samaga Krishna Kumar1.
1Department of Peadiatrics, Center for Clinical and Translational Research, Nationwide Childrens Hospital and Ohio State University, Columbus, OH, United States
Native islets in the pancreas have a rich vascular supply however, the current islet isolation technique completely severs the vasculature to islets. As such, these islets become avascular and many of them die immediately after islet transplantation.
So our aim was to stimulate intra-islet endothelial cells (IIEC) to form peri-islet vessels (PIV) in culture prior to transplantation. In this study, we accelerated the formation of PIV to human islets (Bioengineered islets –BEI) by stimulating the IIEC with the addition of ECGS (a mixture of endothelial growth factors) in a 3D-culture system.
Human islets cells were isolated from donor research pancreases (n=15) and the islets were cultured in a 3D system using collagen-I gel with ECGS containing medium for 14 days. 2D cultured islets and 3D cultured islets without ECGS were used us control groups. Time-lapse microscopy (Cytation 5) was used to monitor islet-derived PIV growth every day for 14 days. To study diabetes reversal, the BEI were implanted in to diabetic nude mice at the subcutaneous site.
Our approach induced cellular sprouting from islets and were positive for endothelial cells (UEA-1) staining.
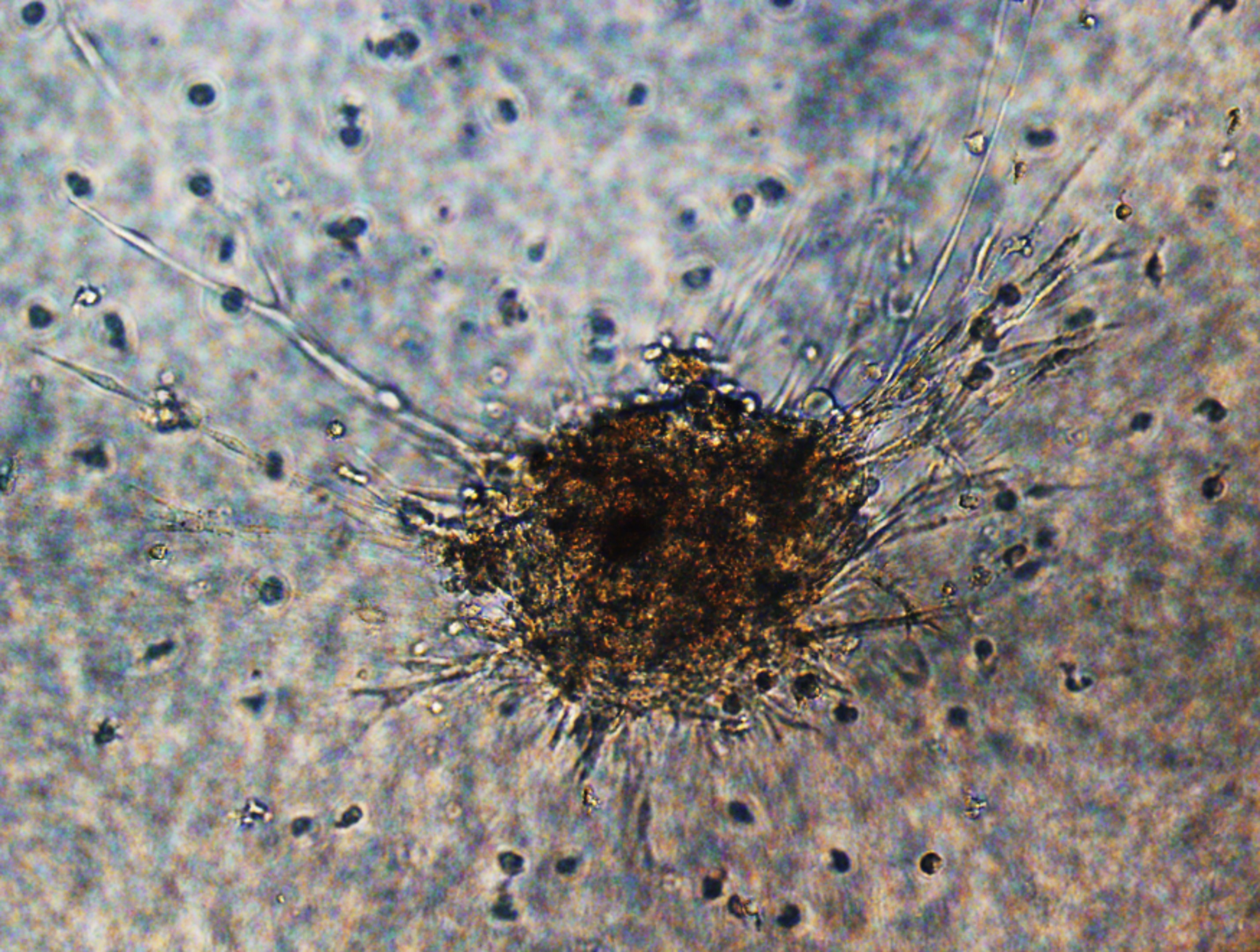
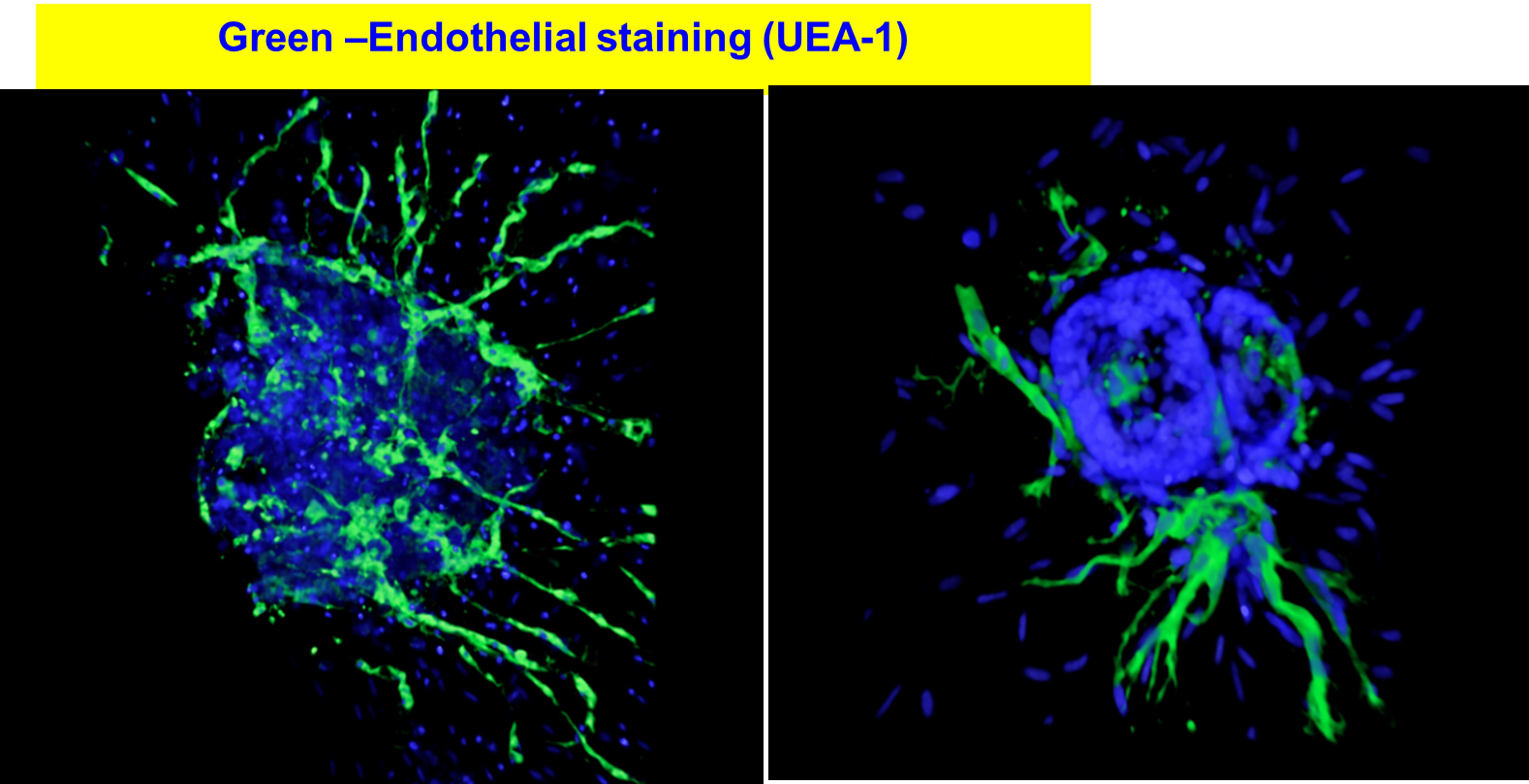
The BEI maintained >90% cell viability, high insulin secretory capacity when compared to control islets and importantly, BEI reversed diabetes in mice. Naked islets cultured in a 2D environment did not form any sprout. However, the presence of ECGS and 3D-culture system induced cellular sprouting from human islets within 7 days. 3D-cultured islets maintained >90% cellular viability after 14 days, whereas 2D-cultured islets displayed disintegration of their borders as well as reduced viability. Statistically significant difference (P<0.05) was observed in number of sprouts when islets were cultured in 3D environment without ECGS.
We demonstrated that 3D matrix support and ECGS are required to elicit successful, spontaneous PIV formation in human islets. ECGS with 3D culture may provide unique avenues to accelerate islet neovascularization following transplantation.