Extracellular vesicles from patients with diabetic nephropathy induce endothelial dysfunction through ICAM-1 and VCAM-1 in an in vitro model
Enrique Montagud-Marrahi1,3, Sergi Torramade-Moix2, Maria José Ramírez-Bajo3, Jordi Rovira3, Elisenda Bañón-Maneus3, Evelyn Hermida1,3, Fritz Diekmann1,3, Marta Palomo2,4,5, Maribel Diaz-Ricart2,5, Pedro Ventura-Aguiar1,3.
1Nephrology and Kidney Transplantation Department, Hospital Clinic of Barcelona, Barcelona, Spain; 2Hematopathology Department. Biomedical Diagnostic Center (CDB), Hospital Clinic of Barcelona, Barcelona, Spain; 3Laboratori Experimental de Nefrologia I Trasplantament (LENIT), Hospital Clinic of Barcelona, Barcelona, Spain; 4Josep Carreras Leukaemia Research Institute, Hospital Clinic of Barcelona / University of Barcelona, Barcelona, Spain; 5Barcelona Endothelium Team, Hospital Clinic of Barcelona / University of Barcelona, Barcelona, Spain
Introduction: Extracellular Vesicles (EVs) are membranous structures produced by cells which contain different cytoplasmic compounds and that have been described as potentially pathogenic elements in endothelial dysfunction (ED) through a modification of the expression of endothelial receptors. The mechanisms of ED in patients with chronic kidney disease (CKD) and diabetes mellitus (DM) are not well defined, although EVs could have a key role.
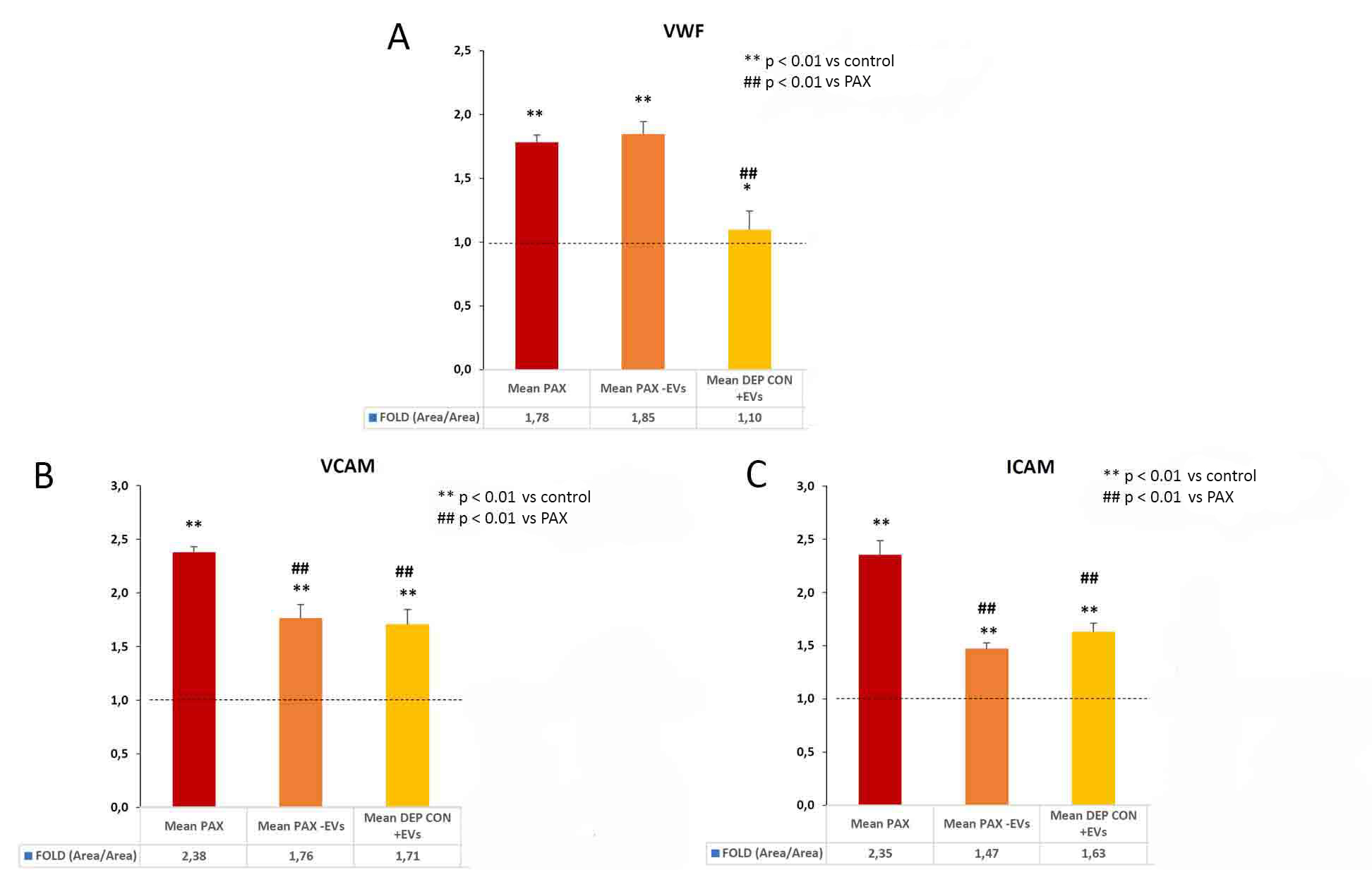
Methods: cross-sectional study with sera from 11 patients with a mean age of 46±7.6 years (45% women) and CKD (eGFR 18±7 mL/min) due to diabetic nephropathy (DM1), grouped into 4 pools. The role of the EVs was studied in an in vitro model of ED with HMEC-1 cells exposed during 72 hours to supplemented medium with: a) patient sera (group 1/red), b) EVs-depleted patient sera (group 2/orange) and c) EVs-depleted control sera in which patient's EVs were added (group 3/yellow). Changes in the expression of vWF and the membrane adhesion receptors VCAM-1 and ICAM-1 were analyzed in the cells exposed to the different conditions, with respect to the healthy donor serum.
Results: the expression of ED markers (vWF, VCAM-1 and ICAM-1) in cells exposed to patient serum (with or without EVs) was higher compared to that observed after exposure to control sera (vWF, p <0.01; Fig. 1A; VCAM-1, p <0.01; Fig. 1B; ICAM-1, p <0.01; Fig. 1C). Moreover, EVs depletion significantly decreases the expression of VCAM-1 and ICAM- 1 with respect to the patient's serum (Fig. 1B, p <0.01 and 1C, p <0.01), but not vWF expression.
Discussion/Conclusions: EVs increase endothelial damage through an increased VCAM-1 and ICAM-1 expression, both being inflammatory markers associated with leukocyte adhesion. Thus, EVs constitute a pathogenic element in ED in patients with DM1 and CKD.