Utility of donor-derived cell-free DNA in assessing simultaneous pancreas-kidney transplantation rejection
Pedro Ventura-Aguiar1,2, Maria Jose Ramirez-Bajo1,2, Navchetan Kaur3, Zachary Demko3, Jordi Rovira1,2, Elisenda Bañón-Maneus1,2, Natalia Hierro1,2, Marta Lazo1,2, David Cucchiari1,2, Nuria Esforzado1,2, Ignacio Revuelta1,2, Gaston Piñeiro1,2, Vicens Torregrosa1,2, Federic Cofan1,2, Constantino Fondevila4, Paul Billings3, Hossein Tabriziani3, Fritz Diekmann1,2.
1Nephrology and Kidney Transplant Department, Hospital Clinic, Barcelona, Spain; 2Laboratori Experimental de Nefrologia i Trasplantament, Fundació Clinic - IDIBAPS, Barcelona, Spain; 3Medical Affairs, Natera, Inc., San Carlos, CA, United States; 4Hepato-Bilio-Pancreatic Surgery and Digestive Transplant Unit, Hospital Clínic, Barcelona, Spain
Introduction: Among solid organ transplants, pancreas transplantation has one of the highest rates of acute rejection within the first 12 months. Graft biopsy, the gold standard, is risky and often not available. Hence, a non-invasive biomarker with good predictive value, such as donor-derived cell free DNA (dd-cfDNA), may be useful in predicting risk of rejection. In this study we sought to analyze the performance of a dd-cfDNA test in patients with simultaneous pancreas kidney (SPK) transplant.
Methods: A retrospective study was conducted from 2017 to 2020 on patients admitted for pancreas graft biopsy at the Hospital Clínic Barcelona. Plasma samples were collected on the day of biopsy. dd-cfDNA fraction was assessed using the ProsperaTM test, a SNP-based mmPCR methodology to predict risk of rejection (including T-cell mediated rejection [TCMR] and antibody mediated rejection [ABMR]).
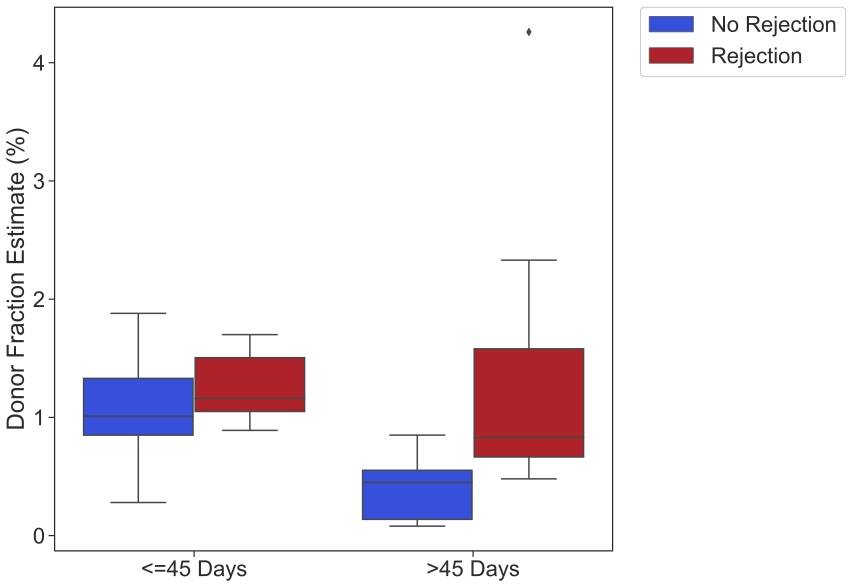
Results: Plasma samples (n=50) were obtained from 37 SPK recipients. Pancreas biopsies were collected from 30 patients. Of the 50 plasma samples, 36 were plasma-biopsy paired and per Banff criteria were categorized as no rejection (n=21) and acute rejection (aTCMR and aABMR; n=15). Median dd-cfDNA levels were significantly elevated in samples with graft rejection diagnosis: 1.11 [0.83-1.66] vs. no rejection 0.65 [0.28-1.00]; p=0.004),especially after 45 days post transplantation (AR: 0.83[0.67-1.59], NR:0.45[0.13-0.55], p=0.004) (Figure 1). The overall performance of the test after 45 days of transplant is as follows: sensitivity 71.4%, specificity 91.6%, PPV: 83.8%, NPV:84.6%.
Conclusion: dd-cfDNA assay can accurately differentiate active rejection from non rejection through a non-invasive, simple blood test in SPK patients as early as 45 days post SPK transplant.
Medical writing support was provided by Nour Al Haj Baddar, Ph.D. from Natera, Inc. at San Carlos, CA.